In modern medicine, treating chronic pain remains a significant clinical challenge. Although numerous molecular targets associated with pain have been identified, efforts to develop non-addictive and safer analgesics have achieved limited success. A primary reason for this is that pain treatment involves more than just targeting a single molecule; it requires addressing the complex interactions between the nervous system, immune system, and target tissues. Traditional drug therapies often fail to account for this systemic dynamic. However, the recent emergence of bioelectronic medicine offers new perspectives and solutions.
This article explores how neuromodulation can directly influence organ function, potentially bypassing the common side effects of traditional drug treatments. Specifically, it focuses on the resurgence of acupuncture in modern medicine and its potential application in managing systemic diseases. By analyzing recent research advancements, we will show how acupuncture activates specific autonomic neural circuits (somatic-autonomic reflexes activating sympathetic/parasympathetic pathways), effectively controlling inflammatory responses and other complex disease states.
Additionally, in collaboration with the NIH-funded SPARC program, this article discusses future directions in acupuncture research and neuromodulation technologies, aiming to provide new therapeutic approaches and scientific insights for clinicians and researchers.
1. Exploring New Therapeutic Strategies in Neural, Immune, and Tissue Interactions
During disease progression, the human body is not just a collection of isolated molecular targets but a system involving the dynamic interaction of the nervous system, immune system, and target tissues. The nervous system not only connects the brain and spinal cord but also interacts closely with the immune system and major organs, regulating their functions through subtle electrical signals. When these signals malfunction, organ dysfunction can occur, causing pain and various conditions such as hypertension, heart disease, urinary incontinence, and gastrointestinal disorders.
Compared to traditional drug therapies, directly transmitting signals to target organs through peripheral nerve stimulation can effectively avoid potential side effects of intermediary processes. In recent years, medical devices that modulate nerve-organ interactions have become increasingly important therapeutic options, known as “bioelectronic medicine.”
However, current commercial devices struggle to replicate the firing patterns of nerve fibers in healthy individuals. This difficulty primarily arises from our limited understanding of the physiological functions of peripheral neural circuits and the distribution and pathways of individual nerve fibers.
Developing nerve stimulation devices based solely on general descriptions of the peripheral nervous system, such as the sympathetic and parasympathetic systems, is inadequate. The nervous system is highly heterogeneous, and every detail can affect the efficacy and safety of treatment. For instance, the vagus nerve in the neck contains approximately 100,000 nerve fibers. The thick A and B fibers have diameters 10-20 times that of the unmyelinated C fibers and have lower electrical stimulation thresholds, requiring much less stimulation than the thin fibers. Current technologies cannot selectively stimulate the thin unmyelinated C fibers without activating the thick fibers, an aspect often overlooked in designing therapeutic strategies.
To develop more effective therapeutic strategies, we must deeply understand the neural mapping and functional interactions of the nervous system and organs at a systemic level, along with the mechanisms of neural regulation during disease progression. This comprehensive understanding can lead to fundamental interventions and treatments, opening new avenues for chronic disease management.
▷ Figure 1. Acupuncture, a characteristic Chinese neuroregulatory method. Source: Wikipedia
Within this medical framework, acupuncture (Fig.1), a traditional Chinese treatment method with over two thousand years of history, enters the modern medical research field with its unique approach. The core idea behind this treatment is that stimulating specific body areas (acupoints) can remotely regulate organ physiological functions. Randomized clinical trials have shown that needling specific body parts (acupoints) can effectively treat gastrointestinal motility disorders, stress urinary incontinence, and chronic pelvic pain.
According to traditional Chinese medicine (TCM) theory, the functional connection between somatic tissues and organs is mediated by “meridians.” However, modern research has yet to provide evidence supporting the physical existence of these “meridians.” 1 Systematically understanding the neural mapping and functional interactions in acupuncture, particularly its regulatory mechanisms during disease progression, will accelerate the modernization of acupuncture research.
2. Acupuncture Drives Specific Autonomic Neural Circuits: The Neural Basis of Treating Inflammation
The neural basis of acupuncture’s anti-inflammatory effects begins with the activation of somatic-autonomic reflexes. This process originates from somatosensory neurons located in the dorsal root ganglia or trigeminal ganglia. Activation of these neurons triggers a cascade of signal transmission through the spinal cord to the brain, ultimately activating peripheral autonomic nervous systems, including the sympathetic and parasympathetic neural circuits that regulate physiological processes (Fig.2).
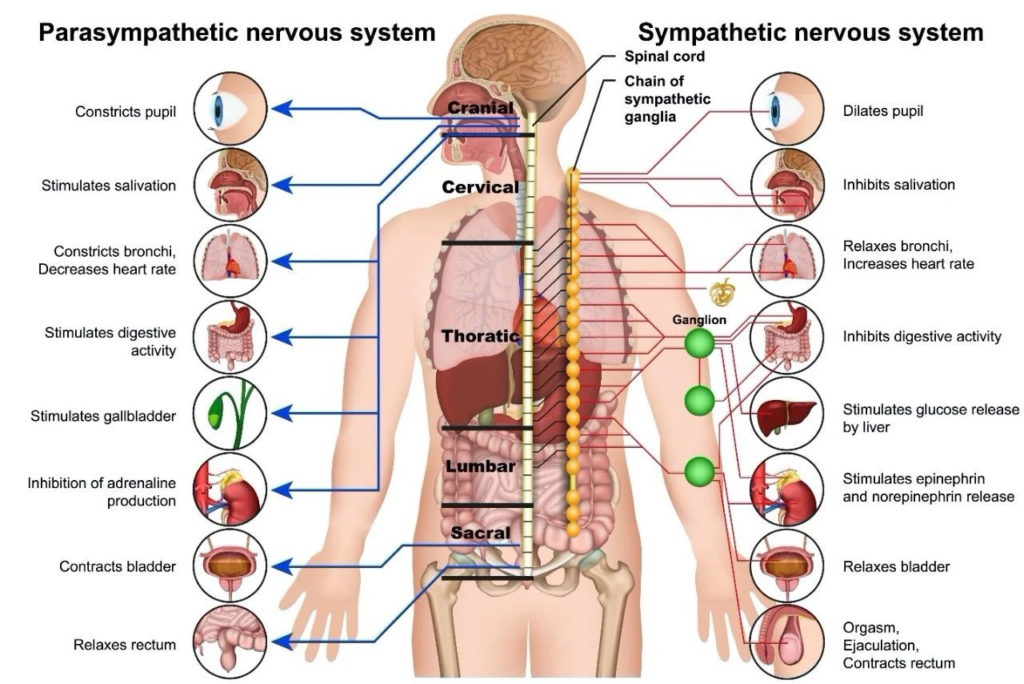
▷ Figure 2. The autonomic nervous system. Source: christopherreeve
(1) Mechanisms of Acupuncture-Activated Neural Reflexes
Recent research has demonstrated the diverse effects of electroacupuncture, which can drive various autonomic neural circuits and dynamically regulate systemic inflammation by modulating immune cell activity 1. For example, in 2000, Tracey and colleagues used electrical stimulation of the cervical vagus nerve to inhibit systemic inflammation (Fig.3). This process is partially achieved by activating splenic sympathetic neurons, although the exact pathway of sympathetic neuron activation remains controversial.
With the advancement of modern molecular and genetic tools, our understanding of somatic-autonomic reflex circuits has become more refined 1. In the study of sympathetic neurons, past methods relied on chemical or surgical approaches, making it challenging to investigate the functional heterogeneity of sympathetic neurons.
The emergence of molecular profiling of sympathetic neurons allows researchers to use cross-genetic tools to label, knock out, or silence relevant neuron subtypes. For instance, in the spleen, the largest immune organ, most sympathetic neurons can be labeled using the NPY-Cre driver. In a model of lipopolysaccharide (LPS)-induced systemic inflammation, it was found that NPY-positive noradrenergic splenic sympathetic neurons function as an endogenous anti-inflammatory system. Their cellular knockout or molecular knockdown exacerbates splenic inflammation.
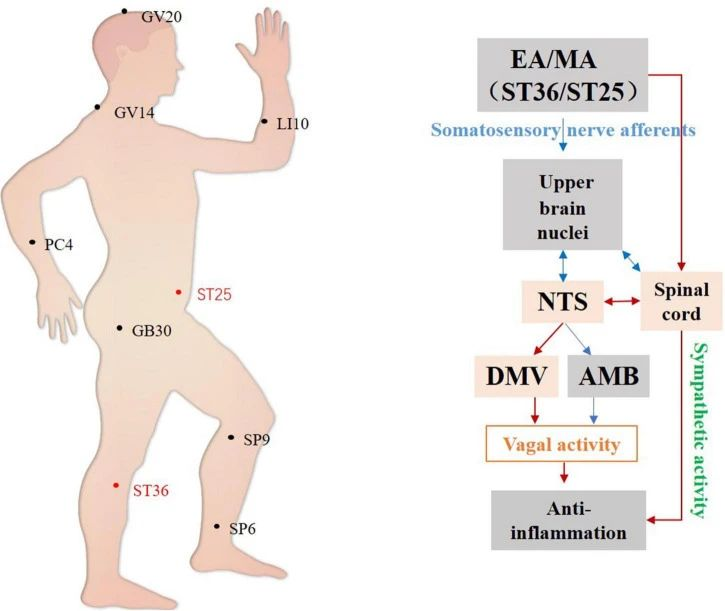
▷ Figure 3. Hypothetical diagram of central autonomic circuits involved in acupuncture-mediated anti-inflammatory effects. Red lines indicate autonomic neural circuits that have been shown to affect acupuncture’s efficacy; blue lines indicate known autonomic physiological pathways that may mediate acupuncture’s effects but are yet to be confirmed. NTS, nucleus tractus solitarius; DMV, dorsal motor nucleus of the vagus; AMB, ambiguous nucleus. Source: Li, Yan-Wei, et al. “The autonomic nervous system: a potential link to the efficacy of acupuncture.” Frontiers in neuroscience 16 (2022): 1038945.
(2) Mechanisms of High-Intensity Electrical Stimulation
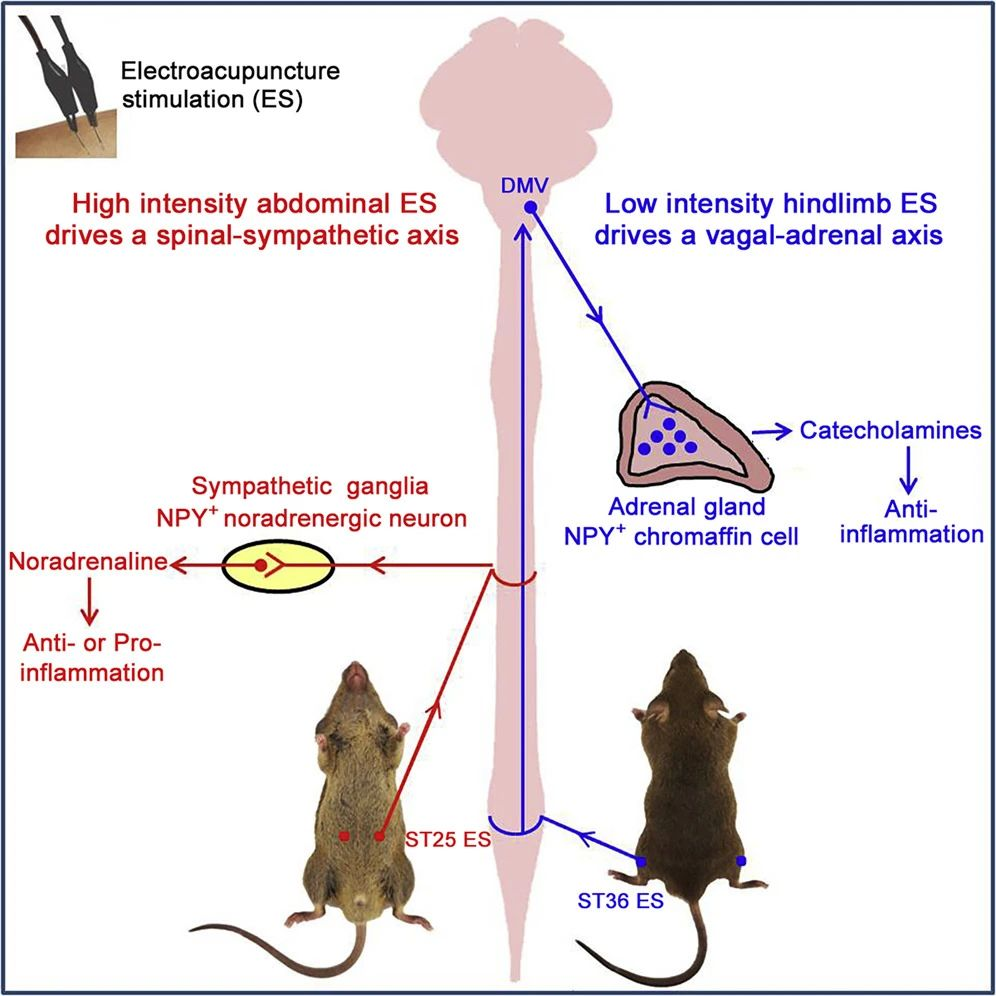
The NPY peptide released from sympathetic neurons can also regulate splenic immune responses. Experiments have shown that high-intensity electrical stimulation (1-3 mA) of the abdominal ST25 acupoint can effectively drive the somatic-spinal-splenic sympathetic circuit, significantly inhibiting LPS-induced systemic inflammation, relying on peripheral NPY-positive splenic noradrenergic neurons. In contrast, low-intensity (0.5 mA) electrical stimulation is insufficient to trigger the same response. High-intensity electrical stimulation of hindlimb acupoints, such as ST36, can also inhibit systemic inflammation through NPY-positive splenic sympathetic neurons rather than vagal reflexes.
The function of this somatic-splenic sympathetic reflex is dynamically regulated by the relative expression levels of anti-inflammatory β2-adrenergic receptors and pro-inflammatory α2-adrenergic receptors. Under normal physiological conditions, splenic immune cells mainly express high levels of β2 receptors; however, after LPS exposure, the enhanced expression of α2 receptors can reduce or even reverse the anti-inflammatory effects of high-intensity electroacupuncture, promoting LPS-induced systemic inflammation.
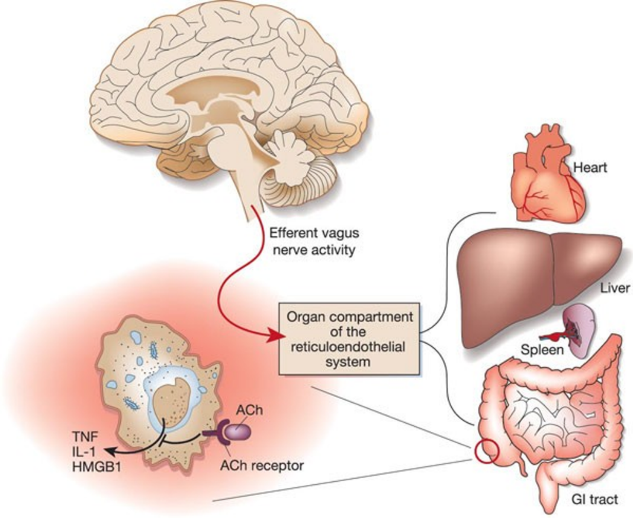
▷ Figure 4. Inhibition of systemic inflammation by electrical stimulation of the cervical vagus nerve, reference 2.
(3) Mechanisms of Low-Intensity Electrical Stimulation
Unlike high-intensity electrical stimulation, which directly activates the somatic-sympathetic neural circuit, recent studies have also revealed how low-intensity electrical stimulation effectively drives the somatic-vagal-adrenal anti-inflammatory neural circuit 1.
Torres-Rosas and colleagues first reported that 4V electroacupuncture stimulation of the ST36 acupoint produces an anti-inflammatory effect, dependent on both vagal reflexes and adrenal catecholamine release. Subsequent studies using genetic markers and cell ablation techniques confirmed the involvement of NPY-positive chromaffin cells in this anti-inflammatory response.
Further experiments demonstrated that low-intensity electroacupuncture (0.5 mA) can evoke a vagal-adrenal neural reflex from the hindlimb ST36 acupoint but not from the abdominal ST25 acupoint 3. Even high-intensity electroacupuncture (3 mA) at the ST25 acupoint failed to activate vagal parasympathetic output neurons in the dorsal motor nucleus of the vagus (DMV), indicating significant acupoint selectivity in the activation of vagal reflexes.
This neural reflex has shown its effectiveness in mitigating LPS-induced systemic inflammation, significantly reducing symptoms and protecting experimental mice from sepsis-induced death. Activating this vagal-adrenal neural reflex can effectively reduce LPS-induced systemic inflammation and protect mice from sepsis mortality. This reflex operates regardless of disease state; electroacupuncture stimulation before or after the peak of LPS-induced cytokine storm produces an anti-inflammatory effect 3. These studies suggest that electroacupuncture can regulate systemic inflammation through different autonomic neural pathways, based on acupoint selection, stimulation intensity, and disease state.
(4) Why Does Acupuncture Drive Specific Autonomic Neural Circuits?
Despite the above research providing valuable insights into the activation mechanisms of spinal circuits such as the somatic-vagal-adrenal axis reflex, the question of “why such reflexes are only activated in limb regions” remains unanswered. Advances in single-cell RNA sequencing of dorsal root ganglia neurons offer hope for addressing this question. Studies have shown distinct profiles of dorsal root ganglia neuron subtypes in the limbs and chest. For example, proprioceptors are significantly amplified in limb dorsal root ganglia compared to the chest.
Recent research by Liu et al. identified a group of neurons enriched in limb dorsal root ganglia, marked by prokineticin receptor 2 (ProkR2-Cre), which specifically drive the somatic-vagal-adrenal axis neural circuit (Fig.5) 4.
A large subpopulation of ProkR2-Cre neurons is myelinated and peptidergic, expressing neurofilament heavy chain protein (NEFH) and calcitonin gene-related peptide (CGRP). They are selectively distributed in deep fascia of limb regions, such as skeletal periosteum, joint ligaments, and fascia surrounding muscles near bones. Activation of these neurons is necessary and sufficient to drive the vagal-adrenal anti-inflammatory reflex 4. The distribution of myelinated ProkR2-Cre nerve fibers can predict the effectiveness of low-intensity electroacupuncture in different body regions for driving the vagal-adrenal anti-inflammatory reflex 4, revealing the neuroanatomical basis of acupuncture driving specific autonomic neural circuits.
▷ Figure 5. Neuroanatomical basis of acupuncture driving the vagal-adrenal anti-inflammatory pathway 3.
(5) What Factors Affect the Effectiveness of Acupuncture?
The effectiveness of acupuncture is greatly influenced by acupoint selection, which displays clear regional specificity along the body’s longitudinal axis. From the perspective of driving the vagal-adrenal axis, effective limb acupoints like ST36 and LI10, compared to ineffective abdominal acupoints and non-acupoint regions in hindlimb muscles, as well as the depth of needling within effective acupoint regions, can all be explained by the differential distribution density of myelinated ProkR2-Cre nerve fibers in various tissues.
In abdominal fascia tissues, such as the peritoneum, myelinated ProkR2-Cre nerve fibers are almost absent, explaining why low-intensity electroacupuncture at the ST25 acupoint fails to effectively activate vagal reflexes or produce anti-inflammatory effects. In contrast, low-intensity electroacupuncture can sufficiently activate this anti-inflammatory reflex in the hindlimb ST36 and forelimb LI10 (Shousanli) acupoint regions, provided the acupuncture needle tip is close to the deep limb fascia rich in peroneal or radial nerves 4.
The sensory nerve distribution along the skin-to-deep-tissue axis indicates that needling depth is another critical factor determining the effectiveness of acupuncture, aligning with ancient Chinese medicine theories. Previous developmental, anatomical, and functional studies have revealed two somatosensory system circuits arranged along the skin and deep tissue 1. Additionally, limb muscles are divided along this axis, with muscle fibers containing slow-twitch type 1 and fast-twitch type 2 fibers, respectively enriched in the internal axial and external peripheral regions 1.
Notably, in the ST36 acupoint region located on the anterior side of the hindlimb, myelinated ProkR2-Cre-labeled nerve fibers that highly express NEFH are densely distributed in the internal region of the tibialis anterior muscle but are sparse in the external region and absent in the epidermis (Figure 6). Therefore, effective treatment in this area requires deeper needle insertion to activate the vagal-adrenal anti-inflammatory reflex.
On the posterior side of the hindlimb, large muscles like the gastrocnemius (GC) and the semitendinosus (SD) in the thigh are similar to the external region of the tibialis anterior, based on common developmental molecular expression and muscle fiber types. These muscles are often used as non-acupoint controls. Unfortunately, myelinated ProkR2-Cre nerve fibers are sparsely distributed within these large posterior hindlimb muscles; therefore, 0.5 mA electrical stimulation in these muscles fails to produce an anti-inflammatory effect 4.
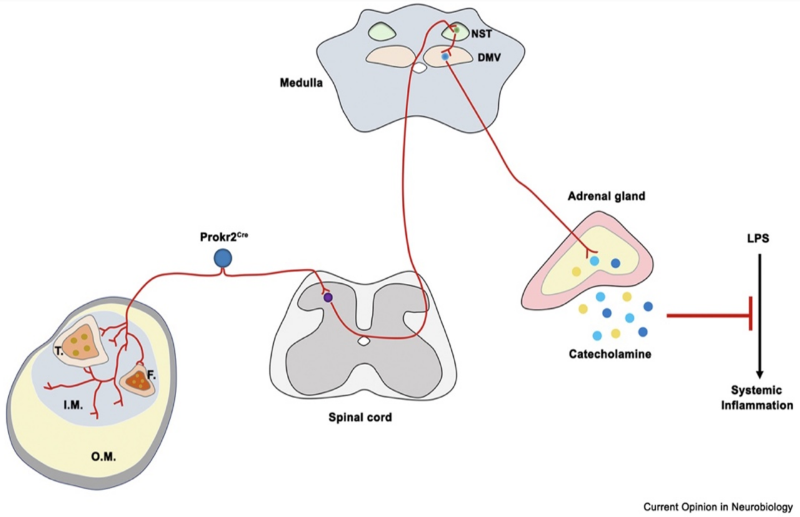
▷ Figure 6. Diagram of the somatic-vagal-adrenal anti-inflammatory axis 1. For cross-sectional illustration of the ST36 region of the hindlimb, light blue and yellow indicate the internal axial (I.M.) and external peripheral (O.M.) regions of the muscle, respectively. T.: tibia. F.: fibula. NST: nucleus tractus solitarius. DMV: dorsal motor nucleus of the vagus.
3. The SPARC Initiative in the United States: A New Impetus for Understanding the Mechanisms of Acupuncture
Understanding and manipulating the complexities of the nervous system is a key area of research in bioelectronic medicine. While individual research teams have made significant advancements, comprehending the interactions between nerves and organs requires broader collaboration and systematic approaches. The NIH’s SPARC (Stimulating Peripheral Activity to Relieve Conditions) initiative was created to meet this need.
The SPARC initiative funds interdisciplinary research projects aimed at achieving precise control over end-organ functions through the development of new neural stimulation devices and protocols.
Researchers under this initiative focus on mapping detailed interactions between nerves and organs and developing neuromodulation devices that effectively control and improve organ functions. They explore new applications for neuromodulation therapies through public-private partnerships and integrate SPARC-funded research data into an online resource called the SPARC Portal, which consolidates neuromodulation data, innovative technologies, and other research resources to promote new scientific discoveries and applications.
▷ https://ncats.nih.gov/research/research-activities/SPARC
In its first phase, SPARC supported the development of new tools and technologies, mapped connections between different neural and organ systems, and created a wealth of public resources available at sparc.science. These efforts provided cutting-edge information and tools to advance bioelectronic medicine.
Building on these achievements, the project has now entered its second phase. This phase focuses on the anatomical and functional connectivity of the human vagus nerve (SPARC-V), creating an open, standardized ecosystem of neuromodulation devices (SPARC-O), challenging the innovation community to demonstrate new capabilities (SPARC-X), and continuing to share data and digital resources through the SPARC Portal. These complementary initiatives aim to promote the development of the next generation of bioelectronic medicine therapies.
SPARC-V (Vagus Nerve Mapping and Physiology): 1. The Reconstructing Vagus Anatomy (REVA) project is creating a more precise and detailed map of the human vagus nerve. 2. The Vagus Nerve Stimulation Parameter Extraction (VESPA) project is determining the physiological effects of modulating vagus nerve activity to discover the best ways to stimulate nerve fibers for specific therapeutic outcomes.
SPARC-O (Open-Source Neuromodulation Technology): The Human Open Research Neural Engineering Technologies (HORNET) project is developing open-source technologies and components to safely and effectively alter neural function.
SPARC-X (Neuromodulation Prize): The Neuromodulation Prize is an exciting challenge competition to inspire proof-of-concept demonstrations of innovative bioelectronic medicine approaches that help patients.
Advancing Bioelectronic Medicine Resources: The SPARC Portal drives the development of bioelectronic medicine by providing open-access digital resources that can be shared, cited, visualized, computed, and used for virtual experiments.
4. Discussion and Prospects for Modern Acupuncture Research
Recent advances have revealed how acupuncture drives disease treatment through specific autonomic neural circuits. For example, to manage cytokine storms, limb acupoints like ST36 are selected for low-intensity electroacupuncture. This method selectively activates the vagal-adrenal anti-inflammatory reflex pathway by targeting myelinated ProkR2-Cre-labeled nerve fiber bundles that innervate the deep fascia of the limbs.
However, the somatic and autonomic nervous systems comprise numerous molecularly and functionally distinct cell types, whose interactions involve complex integrations within the spinal cord and brain. Thus, our current mapping of somatic-autonomic reflex circuits only scratches the surface 1.
For instance, the detailed mechanisms of somatosensory pathways driving most autonomic neural circuits, such as the sympathetic pathways to the stomach, spleen, and kidneys, and the parasympathetic efferent pathways innervating visceral organs, have yet to be fully characterized. Early studies on the analgesic effects of electroacupuncture indicate that stimulation frequency is another crucial parameter 1, but how this parameter influences somatic-autonomic reflexes remains largely unknown.
Additionally, acupuncture is typically applied under pathological conditions in clinical settings, fundamentally different from the normal physiological conditions under which laboratory studies are conducted. For example, visceral diseases can cause referred pain in skin areas, and needling these sensitive skin regions, traditionally known as Ashi points, is paradoxically used to treat visceral diseases 1. However, with central sensitization causing skin hypersensitivity, electroacupuncture-induced somatic-autonomic reflexes may change dramatically, similar to how normally innocuous mechanical stimulation of the skin can become pain-inducing under pathological conditions.
Therefore, studying the plasticity of somatic-autonomic reflexes under various pathological conditions is crucial. This research helps optimize stimulation parameters for acupuncture in remotely treating diseases and provides new insights for therapeutic strategies. Moreover, integrating the SPARC initiative in the United States shows that future research will go beyond mapping the functional and anatomical neural circuits related to acupuncture in mice.
Broadly, we need to resolve the functional and anatomical neural circuit maps related to human acupuncture, building an open, standardized data resource and ecosystem. This includes functional anatomical neural maps, virtual neurofunctional anatomical models, regulatory devices and related parameters, and specific treatment protocols for diseases and symptoms. Achieving this requires interdisciplinary innovation and collaboration among researchers, clinicians, industry, and government sectors.