Torrential rain poured down as a T. rex pressed against a trembling Jeep, its scarlet eyes flashing in the rearview mirror. Meanwhile, in the kitchen, velociraptors’ claws struck the tiles, and people huddled in the corners, holding their breath… The heart-pounding chase scenes in “Jurassic Park” make us ponder:
Had it not been for the meteor that altered Earth’s fate or the ice age that devoured everything, could small mammals like humans, facing these immensely powerful and intelligent giants, truly have established themselves in this jungle?
However, history is always full of dramatic twists. Humans took the stage and competed with the giants not through brute strength, but through the unique intelligence of tool use. It is this intelligence that has allowed us to evolve from vulnerable survivors to the dominant species on Earth, even holding the potential to realize a stunning concept: resurrecting the long-extinct dinosaurs.
▷ Figure 1. Deep Time Cognition Team Homepage. Source: Deep Time Cognition Official Website
Besides dinosaur enthusiasts, there is a group of researchers interested in the relationship between humans and dinosaurs. They belong to DeepTimeCognition, a research team composed of cognitive ethologists, neuroanatomists, and paleontologists, who are dedicated to exploring the development of cognition from an evolutionary perspective.

▷ Osvath M, Němec P, Brusatte SL, Witmer LM. Thought for food: the endothermic brain hypothesis. Trends Cogn Sci. 2024;28(11):998-1010. doi:10.1016/j.tics.2024.08.002.
In November 2024, the team published a study in the journal Trends in Cognitive Sciences, exploring the relationship between “whole-body endothermy” and the evolution of “consciousness.” They observed that dinosaurs and mammals independently evolved endothermic traits, and alongside this change, alterations in neural cognitive structures occurred: the number of neurons surged to twenty times the original number, and new brain structures simultaneously evolved. Through substantial evidence, the research team demonstrated a profound connection between whole-body endothermy and the development of cognitive abilities, highlighting the unique perspectives and significance of studying the brain from the viewpoints of evolutionary biology and comparative biology.
1. How Did Whole-Body Endothermy Evolve?
What exactly is “whole-body endothermy”? It is a physiological trait that enables endothermic animals to maintain a stable body temperature through internal metabolism, without relying on external environmental temperatures. This characteristic requires extremely high metabolic support, with birds and mammals being the most typical representatives among extant species. In terms of neural cognitive functions, birds exhibit astonishing similarities to mammals. This resemblance does not exist in reptiles, despite birds being more closely related to reptiles from an evolutionary perspective.
It can be said that the transition from ectothermy to endothermy is one of the most dramatic changes in the evolutionary history of the brain. As the only two groups of tachymetabolic endotherms on Earth, birds and mammals likely share these cognitive traits because they are essential for supporting their high-energy metabolic lifestyles. This commonality provides important clues for our understanding of the development of cognitive abilities.
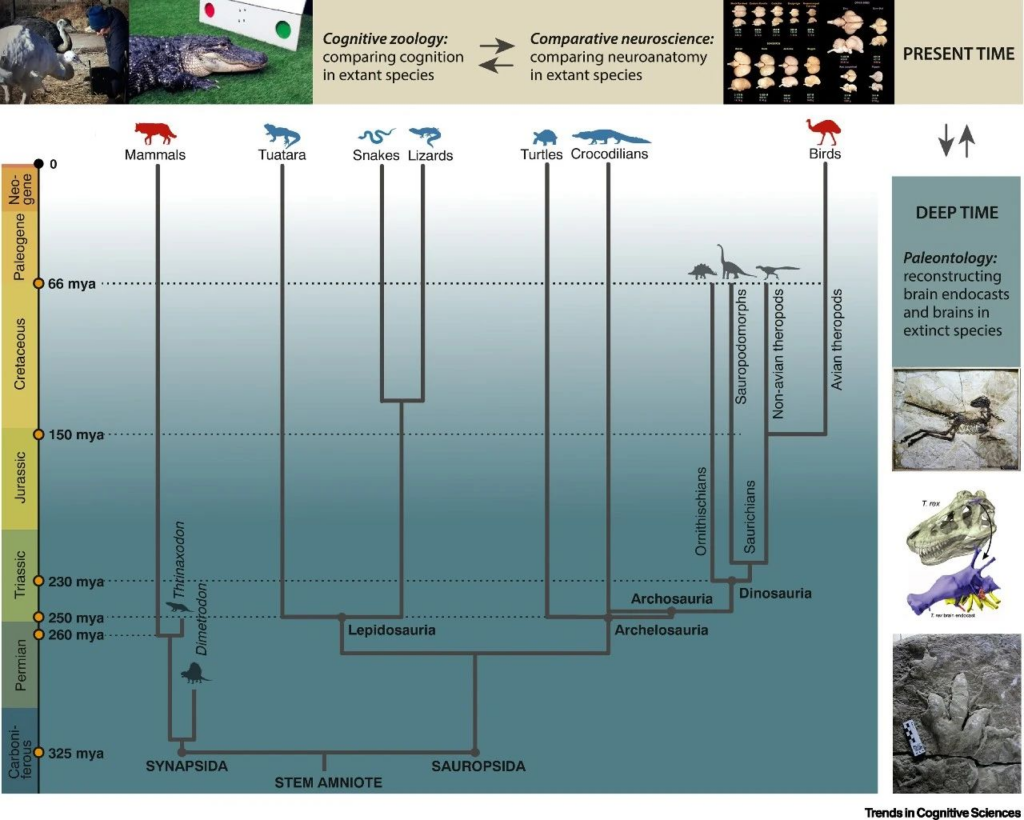
▷ Figure 2. Simplified Evolutionary Diagram and Research Methodology. Source: Trends in Cognitive Sciences
2. What is the Relationship Between Cognition and Whole-Body Endothermy?
“Whole-body endothermy” pertains to energy consumption and biological metabolism, while “cognition” is a core function of the brain. Why are these two seemingly unrelated traits closely linked in evolution? The mere co-occurrence of “whole-body endothermy” and “cognition” may not be sufficiently convincing.
To sustain themselves, organisms must convert external energy into energy that supports their cellular activities and structures; this network of chemical activities is collectively known as “metabolism.” In addition, to prevent collapse, organisms seek out the substances required for metabolism while avoiding potential harm. This balance is primarily maintained through allostasis, which refers to the regulation of stability through change. Allostasis involves not only passively responding to changes but also actively predicting and coping with disturbances. Cognition is a homeostatic regulation system closely connected to an organism’s environment. Therefore, it is believed to be closely related to metabolism [1]:
“Cognition consists of sensory and other information processing mechanisms through which organisms become familiar with, evaluate, and interact effectively with environmental features to meet their survival needs. The most fundamental needs are survival/maintenance, growth/prosperity, and reproduction.”
From this perspective, the nervous system can be viewed as an evolutionary extension for maintaining the organism’s basic allostasis. The emergence of the brain enables organisms to move more quickly and over longer distances, thereby accessing more information and better storing this information in memory.
In this light, the brain is extremely important in energy intake—does this mean that a more developed brain is always better? Such a notion may overlook the “investment” cost associated with brain development. The “expensive brain hypothesis” posits that having a larger brain requires either an increase in total energy intake or a reduction in other high-energy-consuming bodily activities to compensate. In fact, in environments with lower energy availability or highly fluctuating conditions, animals typically have smaller brains, meaning these animals “choose” smaller brains to reduce the “expense” of brain maintenance.
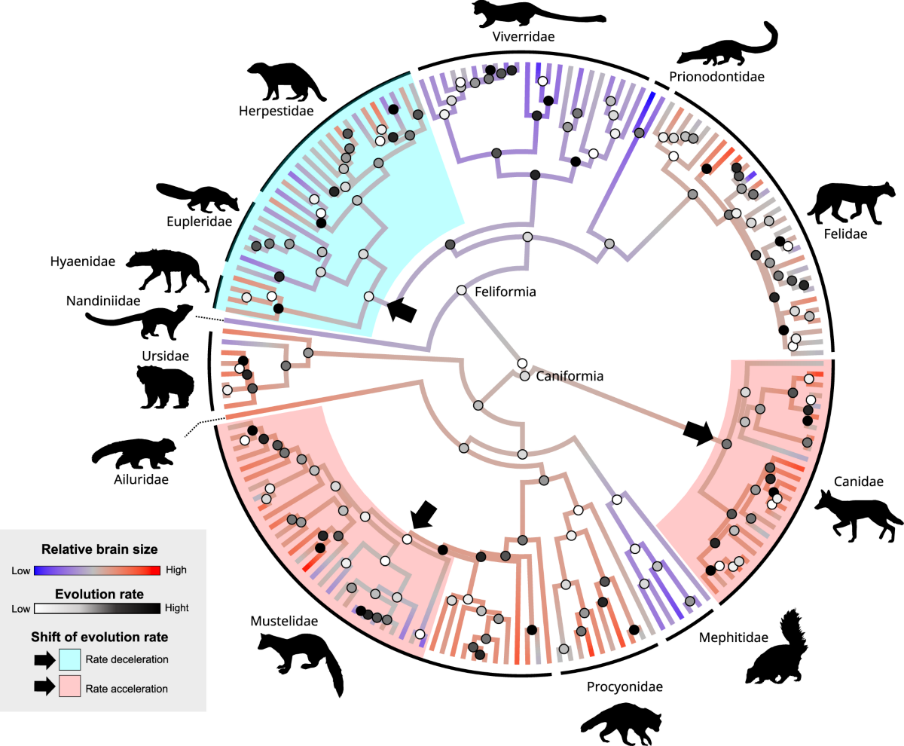
▷ Figure 3. Evolution of Brain Size in Carnivores. Source: Nature
However, when comparing ectothermic and endothermic animals, this linear relationship in brain size does not hold. In endothermic animals, the number of high-energy neurons increases exponentially—representing a change in the cost/benefit ratio of neural tissue. An interesting observation is that in endothermic animals, having a larger brain often correlates with longer lifespans, whereas in ectothermic amniotes, the opposite is true. This implies that in reptiles, investment in neural tissue diverts energy from other bodily activities, accelerating the aging process.
Endothermy fundamentally changes this relationship: the abundance of high-energy neurons not only does not compete with other bodily activities but also supports the maintenance of these activities. For endothermic animals, brain development is a high-risk but higher-reward “investment”: possessing a more neuron-rich and functionally flexible brain incurs energy costs that are negligible compared to the enhancements in brain functionality.
Moreover, the brains of endothermic animals play a crucial role in parental care. Compared to ectothermic animals, endothermic animals exhibit more common and advanced parental care behaviors. The essence of this phenomenon lies in the fact that the energy acquired by endothermic animals not only supports their own bodily activities and brain functions but also meets the needs of other individuals.
3. The Evolutionary Battle Between Brain Development and Energy Consumption: Who Prevails?
By comparing endothermic birds with ectothermic amniotes, we have come to understand the close relationship between “whole-body endothermy” and “cognition.” But how does a qualitative change like brain function occur?
First, we need to identify the differences in cognitive functions supported by these two types of brains and understand the reasons behind these differences.
With the emergence of endothermy, life entered the “age of gluttony.” Prior to this, animals had never consumed as much food as endothermic species do. Existing endothermic animals have food intake (by weight) that is 8 times (mammals) to 11 times (birds) that of equally sized active reptiles [2].
While ectothermic animals also require food to sustain life, grow, develop, and reproduce, endothermic animals additionally depend on food to fuel their high metabolism and generate body heat. Ectothermic animals can survive for extended periods by relying on external heat sources without food, and their lower dependence on food allows them to further conserve energy by reducing foraging activities [3].

▷ Figure 4. Box Turtles in Hibernation. Source: Boxturtles.com
Based on these differences, it can be inferred that the evolution of cognitive functions in endothermic animals primarily serves the purpose of obtaining food. Their high metabolism enables them to forage over greater distances and cope with fluctuations in environmental temperatures. However, high metabolism necessitates such energy-intensive foraging behavior.
At the core of foraging lies an eternal dilemma faced by all mobile organisms: whether to fully exploit existing resources or to explore potentially better resources. This issue is so fundamental that the brain originally evolved to solve it and has since continuously developed in complexity around this theme [4].
In model-free exploration, animals guide their behavior through random searching (assigning equal value to any exploratory action) and stimulus-response associations that rely on habits, occasionally stumbling upon valuable information [5].
In contrast, model-based exploration relies on the animal’s cognitive model of the environment and its goal-directedness. It involves simulating various actions within an environment-based psychological model derived from past experiences, thereby evaluating options and predicting outcomes.
Thus, exploratory foraging transforms from a blind, unknown adventure into a continually optimized search process for energy intake.
4. How Does Endothermy Shape Neural Cognition?
The observed neurocognitive adaptations in birds and mammals support the hypothesis that endothermic brains have evolved primarily to achieve efficient model-based exploration, addressing the energy demands of endothermic animals.
This evolutionary development is manifested in several ways: both birds and mammals have independently evolved larger relative brain sizes [6] and higher neuron densities [7, 8], resulting in significantly enhanced information processing and memory capacities. Compared to reptiles of equivalent body weight, birds and mammals have, on average, 17 times and 9 times more neurons in the telencephalon, respectively, and 45 times and 69 times more neurons in the cerebellum [8].
The expansion of the mammalian telencephalon is closely associated with the emergence of the six-layer neocortex. For example, visual information is organized in a topological and hierarchical manner, forming retinotopic coding. Perceptual information from different sensory modalities is integrated in association areas to create functional modules, such as the limbic system, executive modules, and premotor modules. Similar features are also present in the telencephalon of birds, where the hyperpallium contains multiple topological maps of visual fields [9]. This structure enables rapid and detailed visual analysis but requires more neurons than the non-retinotopic coding found in the visual cortices of reptiles [10]. In both birds and mammals, modular brain regions have become increasingly complex.
Additionally, birds and mammals have convergently evolved complex behavior coordination areas in their telencephalon—the prefrontal cortex (PFC) in mammals and the nidopallium caudolaterale (NCL) in birds [11]. Although these regions are not homologous anatomically, they are highly similar functionally. Both the PFC and NCL receive input from all sensory modalities, spatial and memory information from the hippocampus, and internal state information from the limbic system. They relay this information to the basal ganglia and motor (premotor) structures, thereby mediating goal-directed actions [12], such as inhibiting behaviors and managing working memory as part of a range of executive functions.
Beyond cortical areas, the evolution of the hippocampus in birds and mammals has also become more convergent. While the hippocampus of most vertebrates can create allocentric spatial maps centered on external environments [13], the structure and connectivity of the hippocampus in birds and mammals are evidently more complex. Their enlarged hippocampi receive preprocessed information from the pallium/cortex and possess rich intrinsic axonal collaterals, allowing them to remember numerous locations without extensive training. Birds and mammals may possess integrated cognitive maps, including valence maps associated with specific locations, to enable faster and more efficient memory encoding [14].
In addition to similarities in information organization and encoding, the brains of birds and mammals have independently evolved more complex basal ganglia to achieve finer control of behavioral patterns [15]. The number of neurons in the basal ganglia of birds alone can reach or even exceed the total number of neurons in the entire telencephalon of reptiles of comparable size [7]. Furthermore, to exert more direct control over movement, both birds and mammals have evolved central and downstream excitatory neural pathways in the brain, which are crucial for dexterous motor skills such as food manipulation and extractive foraging [16].
The cerebellum is primarily associated with motor control and the predictive computations required for learning [17]. Amphibians and squamate reptiles have smaller cerebellums with fewer neurons, while crocodilians (turtles and archosaurs) and lepidosaurs have increased cerebellum volume and neuron counts, with birds and mammals exhibiting the most significant expansions [18]. Additionally, birds and mammals have evolved telencephalon-cerebellum pathways, whose development is related to the size of the telencephalon. Although the expansion of the cerebellum in endothermic animals was initially driven by complex terrestrial locomotion and upright posture to facilitate efficient respiration during sustained movement, increasing evidence suggests that the cerebellum plays an important role in various cognitive functions.
The demand for effective foraging has driven the evolution of these functionally similar yet independently evolved brain structures. Beyond more direct sensory-motor control, birds and mammals have also developed larger and more detailed cognitive maps. A key feature of the newly evolved neocortex/cerebellum system is “offline” sensory simulation, allowing animals to evaluate different options through hippocampal cognitive maps before taking action.
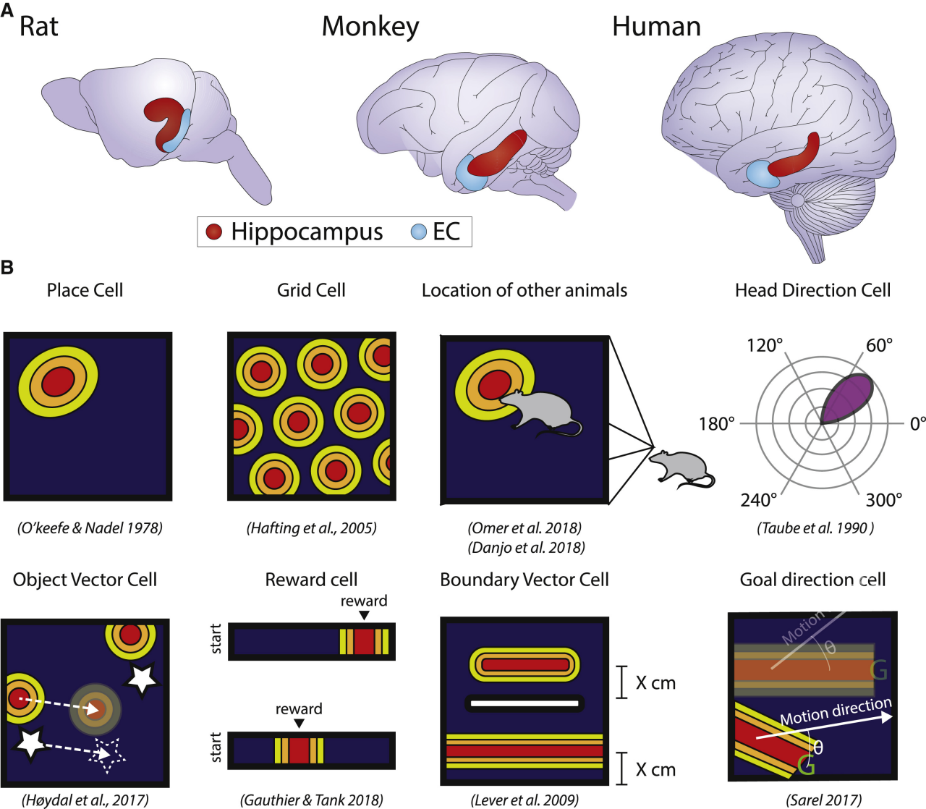
▷ Figure 5. Cells Associated with Hippocampal Cognitive Map Functions. Source: Neuron
Furthermore, the basal ganglia cannot distinguish between perceptual simulations and reality—this may prompt animals to make action decisions based on more energy-efficient simulated scenarios. The prefrontal cortex (PFC) and nidopallium caudolaterale (NCL) play crucial roles in balancing outcome-oriented memory with habitual behavior, deciding whether to inhibit instinctual tendencies or replace old experiences with new knowledge. These top-down processes help make more informed decisions and are believed to have partially evolved to increase caloric intake.
5. How to Deeply Understand Neural Cognition Through the Dimension of Time
The Deep Time Cognition team has combined differences in the survival strategies of ectothermic and endothermic animals with neuroanatomical evidence, uncovering the simultaneous evolutionary emergence of the cognitive revolution and endothermy. Through cognitive neuroscience research, they have elucidated the evolutionary processes of the brain and the mechanisms of information perception. For an in-depth study of the information processing methods of ectothermic and endothermic animals, they recommend conducting comparative neuroscience research. This research should focus on species located at critical nodes of system development.
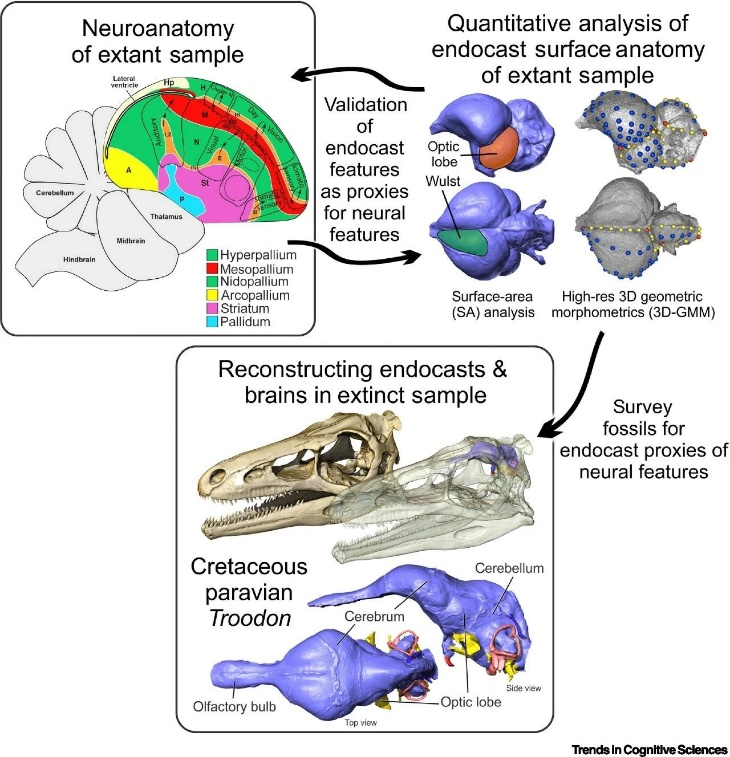
▷ Figure 6. Workflow for Deep Temporal and Spatial Interpretation of Neural Cognition. Source: Trends in Cognitive Sciences
Among archosaurs, all species except endothermic birds have gone extinct. However, within reptiles, numerous extant species remain, including ectothermic animals (lizards, snakes, tuataras, turtles, and crocodilians) and endothermic animals (birds). In reptiles, endothermy likely appeared within the Archosauria clade, whose current representative species include crocodilians (ectothermic) and birds (endothermic). Crocodilians largely retain the neuroanatomical structures of ancient archosaurs. These ancestral features are shared by crocodilians, non-avian dinosaurs, and birds.
Palaeognaths represent the most primitive birds discovered in neuroanatomical studies and share many similarities with non-avian paravians (Paravians). They also have the lowest metabolic rates among birds, which may reflect their primitive energy regulation methods. By comparing the cognitive abilities of crocodilians with those of palaeognaths, researchers can approximate the evolutionary transition of ancient archosaurs from ectothermy to early, fully high-level metabolic endothermy. Although research in this area is still limited, existing findings suggest that palaeognaths already possess robust social cognitive abilities.
Studying other ectothermic reptiles beyond archosaurs is also important. Neurocognitive adaptive changes that appeared even earlier in some archosaurs may have facilitated the emergence of endothermy. For example, specialized structures in crocodilians, such as the rudimentary nidopallium caudolaterale (NCL) and projections between the forebrain and cerebellum, play significant roles in endothermic animals. It is necessary to determine whether these structures are unique to ancient archosaurs.
6. Conclusion
The progressively increasing energy demands throughout evolutionary history are reflected in cognitive and neuroanatomical structures. This shift aligns with the trend from ectothermy to endothermy and has occurred independently along the evolutionary paths of birds and mammals. Cognitive abilities support the survival of organisms, while elevated metabolic rates promote an increase in neuron numbers and the emergence of new brain structures. These new structures, in turn, enhance cognitive efficiency. In essence, how animals “eat” and how they “think” are intricately interconnected processes.
The Deep Time Cognition team combined differences in the survival strategies of ectothermic and endothermic animals with neuroanatomical evidence, uncovering the simultaneous evolutionary emergence of the cognitive revolution and endothermy. Through cognitive neuroscience research, they revealed the evolutionary processes of the brain and the mechanisms of information perception.
Tracing the evolution of the brain over geological timescales not only helps us explore the nature of “intelligence” but also prompts us to reassess our relationships with other species in the natural world. To better understand this cognitive shift, comprehensive comparative studies of cognition and neuroanatomy are necessary for extant ectothermic and endothermic animals located at critical points in system development. Although current research tasks are challenging and many core questions remain unresolved, the time is ripe for conducting such studies.
Questions Yet to Be Addressed:
• What are the primary cognitive differences between existing ectothermic and endothermic amniotes?
• How are cognitive abilities and brain anatomical structures related within and between ectothermic and endothermic animals?
• Can we identify brain region correlations unique to endothermic animals in extinct species?
• How have cognitive abilities gradually evolved during the process of endothermy?
[1] LYON P. Of what is “minimal cognition” the half-baked version? [J]. Adaptive Behavior, 2020, 28(6): 407-24.
[2] NAGY K A. Food requirements of wild animals: predictive equations for free-living mammals, reptiles, and birds, F, 2001 [C].
[3] CAREAU V, KILLEN S S, METCALFE N B. Adding Fuel to the “Fire of Life”: Energy Budgets across Levels of Variation in Ectotherms and Endotherms [M]. Integrative Organismal Biology. 2014: 219-33.
[4] CISEK P. Evolution of behavioural control from chordates to primates [J]. Philosophical Transactions of the Royal Society B: Biological Sciences, 2021, 377(1844): 20200522.
[5] HILLS T T, TODD P M, LAZER D, et al. Exploration versus exploitation in space, mind, and society [J]. Trends in Cognitive Sciences, 2015, 19(1): 46-54.
[6] SMAERS J B, ROTHMAN R S, HUDSON D R, et al. The evolution of mammalian brain size [J]. Sci Adv, 2021, 7(18).
[7] OLKOWICZ S, KOCOUREK M, LUČAN R K, et al. Birds have primate-like numbers of neurons in the forebrain [J]. Proc Natl Acad Sci U S A, 2016, 113(26): 7255-60.
[8] KVERKOVÁ K, MARHOUNOVÁ L, POLONYIOVÁ A, et al. The evolution of brain neuron numbers in amniotes [J]. Proc Natl Acad Sci U S A, 2022, 119(11): e2121624119.
[9] BISCHOF H J, ECKMEIER D, KEARY N, et al. Multiple Visual Field Representations in the Visual Wulst of a Laterally Eyed Bird, the Zebra Finch (Taeniopygia guttata) [J]. PLoS One, 2016, 11(5): e0154927.
[10] KNUDSEN E I. Evolution of neural processing for visual perception in vertebrates [J]. J Comp Neurol, 2020, 528(17): 2888-901.
[11] MILLER E K, COHEN J D. An integrative theory of prefrontal cortex function [J]. Annu Rev Neurosci, 2001, 24: 167-202.
[12] KERSTEN Y, MOLL F W, ERDLE S, et al. Input and Output Connections of the Crow Nidopallium Caudolaterale [J]. eneuro, 2024, 11(4): ENEURO.0098-24.2024.
[13] MURRAY E A, WISE S P, GRAHAM K S. The evolution of memory systems: Ancestors, anatomy, and adaptations [M]. New York, NY, US: Oxford University Press, 2017.
[14] MADISON F N, BINGMAN V P, SMULDERS T V, et al. A bird’s eye view of the hippocampus beyond space: Behavioral, neuroanatomical, and neuroendocrine perspectives [J]. Horm Behav, 2024, 157: 105451.
[15] FROST-NYLÉN J, THOMPSON W S, ROBERTSON B, et al. The Basal Ganglia Downstream Control of Action – An Evolutionarily Conserved Strategy [J]. Curr Neuropharmacol, 2024, 22(9): 1419-30.
[16] TEN DONKELAAR H J. Evolution of Motor Systems: Corticospinal, Reticulospinal, Rubrospinal and Vestibulospinal Systems [M]//BINDER M D, HIROKAWA N, WINDHORST U. Encyclopedia of Neuroscience. Berlin, Heidelberg; Springer Berlin Heidelberg. 2009: 1248-54.
[17] TANAKA H, ISHIKAWA T, LEE J, et al. The Cerebro-Cerebellum as a Locus of Forward Model: A Review [J]. Front Syst Neurosci, 2020, 14: 19.
[18] HEUER K, TRAUT N, DE SOUSA A A, et al. Diversity and evolution of cerebellar folding in mammals [J]. Elife, 2023, 12.