1. Principles
(1) Physical Basis
The sounds we hear in our daily lives are all perceptible sound waves to humans, with frequencies ranging from 20 Hz to 20,000 Hz. Focused Ultrasound (FUS), however, utilizes ultrasound waves of much higher frequencies, far beyond the range of human hearing.
During the propagation of ultrasound waves, interference occurs, meaning the waves can mutually enhance or cancel each other out. By strategically arranging multiple ultrasound transducers, we can harness this interference to concentrate the energy of ultrasound waves at a specific focal point. This technique of focusing using ultrasound interference is known as FUS.
In an FUS system, each transducer can independently control the phase of the sound wave. By precisely calculating the phase of each transducer, a desired focal point can be produced. However, in practical applications, the shape and size of the focal point are also influenced by other factors, such as the propagation characteristics of the sound waves in different materials and the acoustic properties of the materials as they vary with temperature and frequency.
To overcome these issues, some FUS systems adopt “dual-mode ultrasound” technology. This involves the simultaneous use of a separate probe for ultrasound imaging while therapeutic ultrasound is being applied, allowing real-time monitoring of the focal point’s position and size and timely adjustment of focusing parameters to optimize therapeutic effects. This technology is currently used in the treatment of localized organ diseases such as the prostate.
The structural design of the ultrasound probe is also crucial for FUS. Different geometric configurations can produce different ultrasound beam shapes, making them suitable for various applications. In neurosurgery, tightly spaced, unidirectional transducer arrays are typically used, imaging along a straight path through small openings in the skull. Besides the geometric configuration of the transducer array, the parameters of the ultrasound waves themselves, such as frequency and amplitude, can also be adjusted. To avoid excessive heat generation, ultrasound is usually delivered in pulses, with the pulse repetition frequency and pulse duration also being adjustable.
(2) Biological Effects
When ultrasound waves penetrate biological tissues, they induce a series of complex physical processes, which can be broadly categorized into thermal and non-thermal effects.
The temperature rise induced by ultrasound in tissues primarily depends on the intensity of the sound waves and the tissue’s absorption properties. Generally, the higher the ultrasound frequency, the shallower the penetration depth but the higher the resolution. This means a balance must be struck between penetration depth, resolution, and frequency. When ultrasound generates heat within tissues, the tissue’s impedance and thermal conductivity properties affect the diffusion of heat. Physiological cooling mechanisms, such as blood perfusion and thermal diffusion, also play significant roles in the tissue heating process.
High-Intensity Focused Ultrasound (HIFU) can generate temperatures within tissues high enough to alter protein structures and coagulate tissues. It has been clinically applied to ablate kidney stones, tumors, and treat certain brain lesions causing movement disorders. In contrast, Low-Intensity Focused Ultrasound (LIFU) induces temperature changes within the normal physiological range, avoiding irreversible damage.
The non-thermal effects of FUS include mechanical forces, radiation forces, and some organ-specific effects, such as reversible opening of the blood-brain barrier and altering neuronal membrane potentials.
The mechanical effects of FUS are evident in its ability to directly act on certain mechanosensitive ion channels and proteins, including sodium and potassium channels, thereby altering the state of neurons. High-intensity ultrasound can also physically tear tissues, but its safety is challenging to assess.
Additionally, when ultrasound intensity is sufficiently high, cavitation occurs. Cavitation refers to the growth and collapse of microbubbles during the compression and expansion phases of sound waves. The threshold for cavitation depends on factors such as sound wave frequency, temperature, and pressure. Microbubble nuclei are needed to initiate cavitation, serving as the starting points for the growth, oscillation (stable cavitation), or violent collapse (inertial cavitation) of microbubbles. In HIFU, gases released due to thermal effects can become the primary source of these microbubble nuclei. Cavitation can influence cell membrane potentials and induce micro-streaming, forming turbulence that affects surrounding cells.
In summary, FUS can produce thermal and non-thermal effects in biological tissues, with distinct impacts. HIFU can raise tissue temperatures to 43-60 degrees Celsius, causing time-dependent damage and, at higher intensities, immediate tissue damage. This damage is mainly achieved through thermal and cavitation effects. With advancements in non-invasive temperature monitoring technology, MRI-assisted HIFU therapy has gained widespread application, allowing precise control of lesion size and ensuring safety.
Conversely, LIFU can induce reversible neurophysiological responses, such as increasing or decreasing neuronal firing rates and conduction velocity, inhibiting visual and somatosensory evoked potentials, EEG, and epileptic seizures. The exact mechanisms of LIFU remain uncertain and may involve thermal effects, mechanical effects, and changes in ion channel activity, warranting further research.
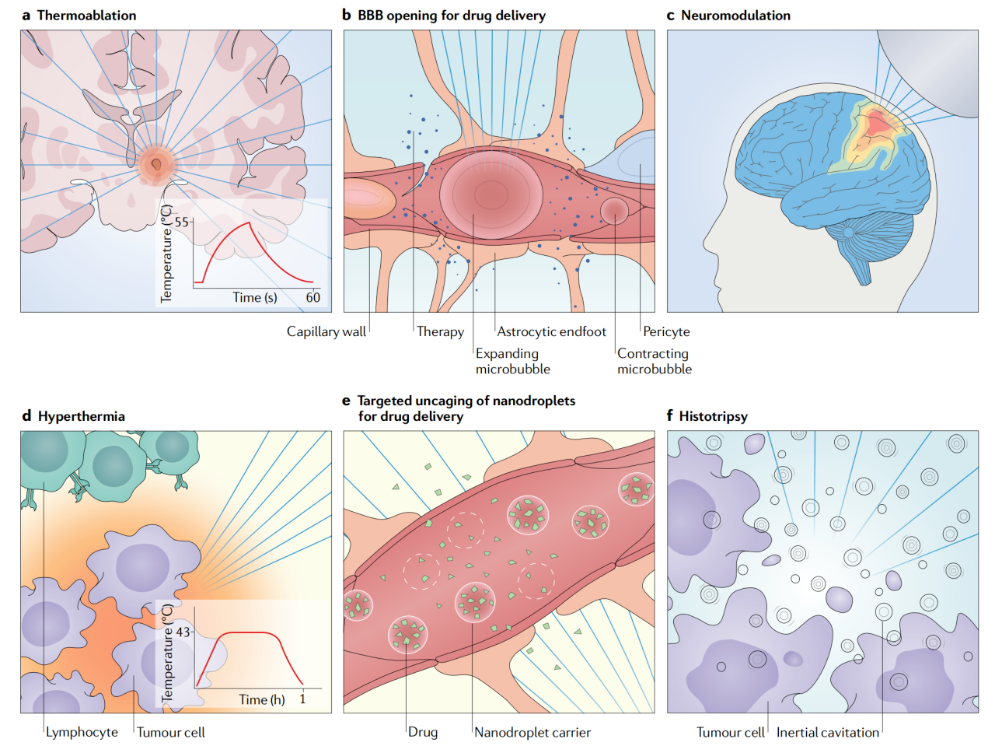
▷ Figure 1. Biological effects of FUS. Source: Meng, Ying, Kullervo Hynynen, and Nir Lipsman. “Applications of focused ultrasound in the brain: from thermoablation to drug delivery.” Nature Reviews Neurology 17.1 (2021): 7-22.
2. Technological Development
In 1935, Gruetzmacher designed a curved quartz plate that could focus ultrasound waves to a single point, leading to the birth of the first FUS transducer. Eight years later, Lynn and colleagues at Columbia University in the United States first reported the application of FUS in the brain during animal experiments. They discovered that by instantly raising HIFU to its maximum intensity, the effects at the focal point could be maximized while minimizing damage to nearby areas.
Despite the technological limitations at the time, these findings established HIFU as a feasible method for creating a precise focal point while reducing damage along the path. They also found that surface and along-the-path damage were inversely proportional to the distance from the focal point, suggesting that the technology might be more suitable for targeting deep brain areas. Additionally, using lower frequencies could reduce absorption and heating of surface tissues, favoring absorption at the focal point. They also found that focused ultrasound could create reversible nerve damage, with ganglion cells being more susceptible than glial cells and blood vessels. These findings laid the groundwork for the subsequent development of FUS by demonstrating its ability to produce safe, reversible effects in biological tissues.
Subsequently, William Fry and Francis Fry from the University of Illinois further advanced FUS technology. Early studies showed that focused ultrasound could damage surface tissues like the scalp and skull, affecting the focus. To address this issue, the Fry brothers decided to apply focused ultrasound directly to the dura mater through craniotomy.
In 1954, the Fry research team published a seminal paper describing their method of targeting deep brain structures using a device with four focused ultrasound beams (see Figure 2). This device could be used in conjunction with stereotactic equipment, demonstrating for the first time the effectiveness of combining focused ultrasound with stereotactic techniques in animal models. They successfully ablated the thalamus and internal capsule in 31 cats, with histological examination showing cellular changes in the target area within two hours of exposure. Unlike Lynn’s findings, this experiment primarily damaged nerve fibers while leaving the neuronal cell bodies in the target area largely unaffected. Additionally, there was no significant damage to blood vessels and surrounding tissues.
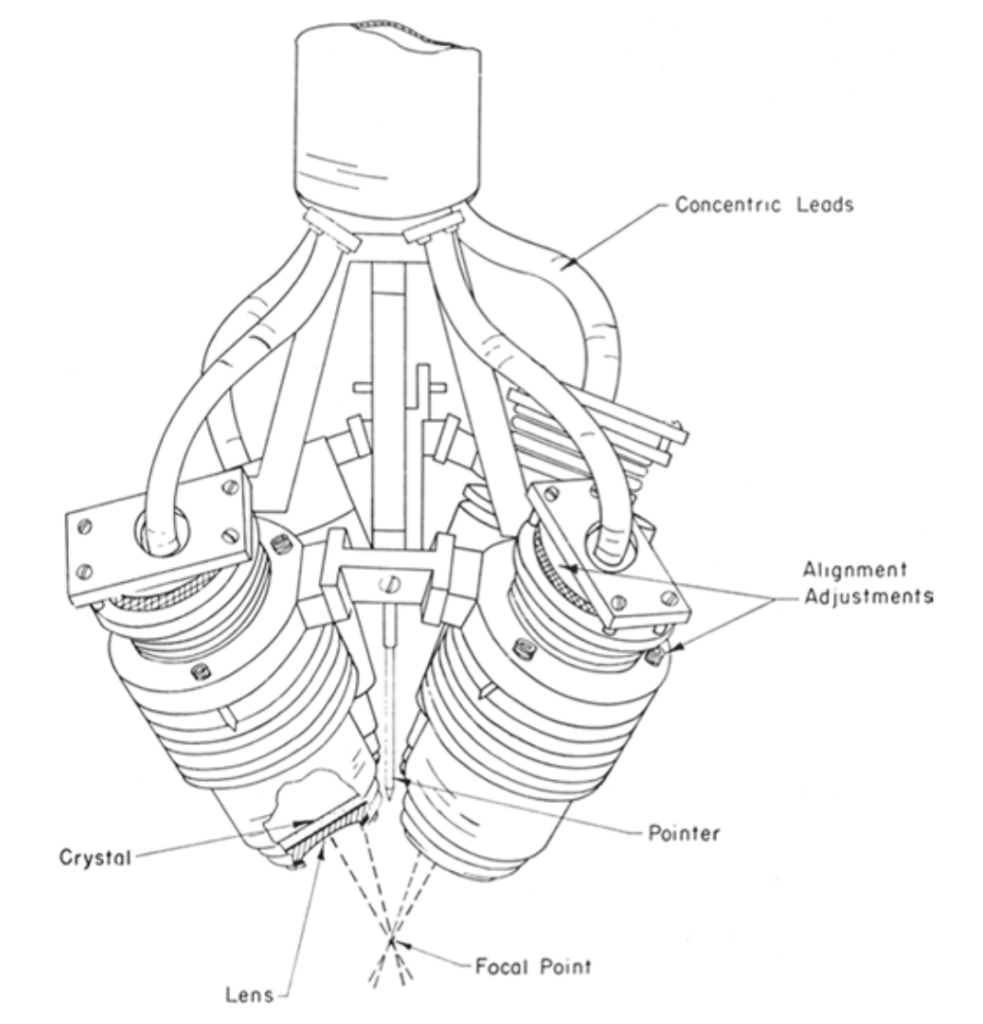
▷ Figure 2. The four-beam focused ultrasound device used by the Frys. Source: Harary, Maya, et al. “Focused ultrasound in neurosurgery: a historical perspective.” Neurosurgical Focus 44.2 (2018): E2.
Meanwhile, the Fry team used precise focused ultrasound stimulation of the lateral geniculate body to temporarily suppress the brain’s response to retinal flash stimuli. Specifically, electrodes were placed on the visual cortex to measure the brain’s electrophysiological response to light stimuli. During focused ultrasound exposure, the amplitude of these evoked potentials decreased to less than one-third of the baseline value. Surprisingly, once the ultrasound stimulation ceased, these electrophysiological indicators returned to their original levels within 30 minutes. More importantly, this dose of focused ultrasound did not cause any observable histological damage to the underlying neural tissue. This discovery pioneered a new concept: FUS neuromodulation.
After achieving success in animal experiments, the Fry laboratory collaborated with the Neurosurgery Department at the University of Iowa to apply FUS in human neurosurgery. They targeted deep brain regions in patients with Parkinson’s disease, attempting to treat tremors and rigidity with FUS. In 1960, Meyers and Fry published a treatment study involving 48 patients, demonstrating the therapeutic effects of FUS on Parkinsonian tremors and rigidity.
By the latter half of the 20th century, the therapeutic potential of FUS had gradually gained recognition. However, to avoid damage and distortion to surface tissues when passing through the skull, craniotomy was necessary, making the procedure still invasive. For FUS to further advance, two critical issues needed to be addressed: transcranial focusing and real-time monitoring.
To achieve transcranial focusing, FUS had to overcome two major obstacles: local overheating of the skull and beam propagation distortion due to tissue inhomogeneity. Bone absorbs ultrasound waves 30-60 times more than soft tissue. Early experiments found that the interaction between ultrasound waves and the skull led to rapid local heating of the skull, limiting the safe level of energy that could be applied. This issue was eventually resolved by using low-frequency hemispherical transducers and actively cooling the scalp. Low frequencies reduced surface absorption, while hemispherical transducers distributed local heating over a larger surface area, and scalp cooling prevented excessive heating.
Additionally, beam propagation and focusing were significantly distorted due to the acoustic impedance mismatch between bone and brain, as well as individual variations in skull shape, thickness, and the ratio of cortical bone to marrow. Until the early 1990s, this problem remained unresolved. The emergence of phased array technology, which corrects for delays and changes encountered during wave propagation by applying different phase shifts to each element, finally enabled precise targeting previously achievable only through craniotomy. Coupled with acoustic feedback techniques to accurately measure phase shifts caused by the human skull, focused ultrasound technology overcame this critical obstacle. These groundbreaking advancements laid the foundation for the modern development of focused ultrasound, making completely non-invasive treatment of deep brain structures possible.
Early applications of HIFU thermal ablation were primarily by surgeons for treating prostate, urinary system, breast, and gynecological tumors. In these applications, physicians could use diagnostic ultrasound to guide and monitor the treatment process in real-time. However, in neurosurgical applications, the skull impeded ultrasound imaging of internal tissue changes. In the late 1980s and early 1990s, Dr. Jolesz’s team pioneered the use of intraoperative magnetic resonance imaging (MRI) to address this issue. Subsequently, they turned their attention to using MR thermometry to monitor temperature changes within the brain in real-time during focused ultrasound treatment.
By the late 1990s, the Jolesz team discovered that low-power FUS could raise the temperature of the target area to 40-42 degrees Celsius without causing damage. This sub-threshold ultrasound exposure generated a thermal signal that could be located and targeted using MR thermometry, preparing for subsequent high-power ablative exposure. In the following years, Jolesz and colleagues focused on characterizing the thermodynamics, ultimately achieving the prediction of lesion size after continuous exposure and real-time monitoring of the thermal damage process.
3. Clinical Applications
(1) HIFU-based Thermal Ablation Therapy
HIFU can produce therapeutic effects by raising the temperature of the target tissue. When the temperature increases to 40-45 degrees Celsius, it can enhance the sensitivity of tumors to radiotherapy or aid in the release of drugs from thermosensitive liposomes. When the temperature exceeds 56 degrees Celsius, it causes tissue denaturation and necrosis.
For common tremor disorders, such as essential tremor, focused ultrasound can target and ablate key areas like the ventral intermediate nucleus (VIM) of the thalamus or the cerebellar-thalamic tract (CTT), effectively alleviating patients’ tremor symptoms. Numerous clinical studies have confirmed that unilateral FUS surgery targeting the VIM or CTT significantly improves patients’ tremor and quality of life, with most adverse effects, such as sensory disturbances and gait abnormalities, being temporary.
In Parkinson’s disease, focused ultrasound also offers multiple target options. For patients primarily exhibiting tremors, ablation of the VIM can be chosen; for motor disorders, the subthalamic nucleus (STN) or globus pallidus internus (GPi) can be targeted; for motor complications, the pallidothalamic tract (PTT) can be targeted. These FUS surgeries effectively improve motor symptoms in Parkinson’s patients, although adverse effects such as speech disorders may occur.
Additionally, focused ultrasound has applications in treating psychiatric disorders like obsessive-compulsive disorder (OCD) and depression. By ablating the anterior limb of the internal capsule (ALIC), it can effectively alleviate symptoms such as obsessive thoughts, depression, and anxiety without causing cognitive decline.
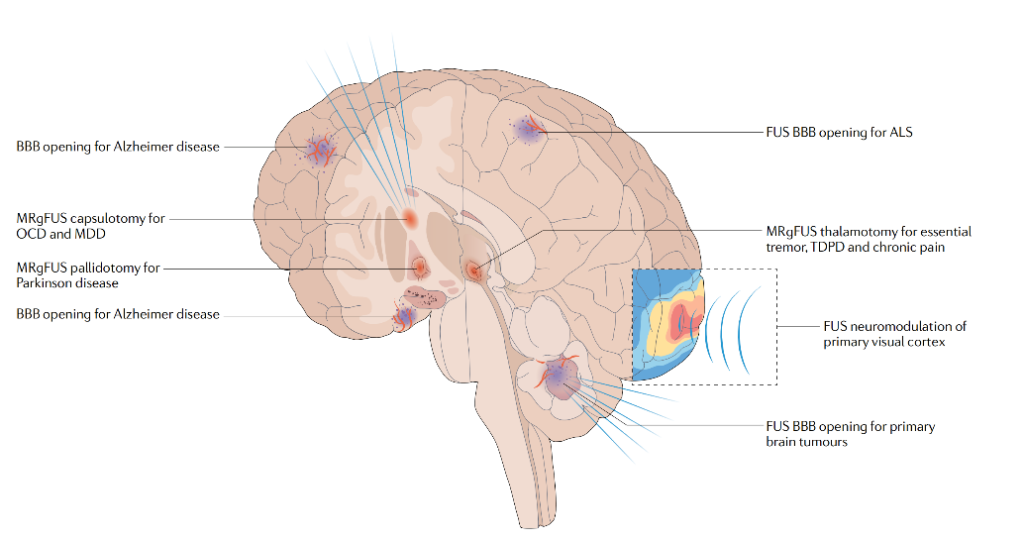
▷ Figure 3. Applications of FUS in the human brain. Source: Meng, Ying, Kullervo Hynynen, and Nir Lipsman. “Applications of focused ultrasound in the brain: from thermoablation to drug delivery.” Nature Reviews Neurology 17.1 (2021): 7-22.
(2) Opening the Blood-Brain Barrier
The blood-brain barrier (BBB) is a barrier formed by the walls of brain capillaries, glial cells, and the choroid plexus. Its main function is to regulate the entry and exit of substances into the brain, maintaining the brain’s stable environment. Although the BBB blocks harmful substances from entering the brain, it also hinders the entry of drugs, especially large-molecule drugs, for disease treatment.
Research has found that low-intensity focused ultrasound (LIFU) can safely and reversibly open the BBB. After injecting microbubbles, ultrasound causes these microbubbles to oscillate, temporarily disrupting the tight junctions of the BBB and allowing better drug penetration into the brain. This method has been shown in animal experiments to effectively enhance the treatment of neurological diseases such as brain tumors, Parkinson’s disease, and Alzheimer’s disease.
Furthermore, this technique of opening the BBB can non-invasively release biomarkers such as phosphorylated tau protein into the blood, aiding in the early diagnosis and monitoring of neurodegenerative diseases and brain tumors. It can also modulate the neuroimmune system to achieve therapeutic effects, such as reducing amyloid plaques and hyperphosphorylated tau protein in Alzheimer’s disease models, promoting adult neurogenesis, and altering the tumor microenvironment.
(3) LIFU-based Neuromodulation
In addition to opening the BBB, LIFU can precisely modulate neural activity in specific brain regions by altering the permeability of neuronal cell membranes and activating ion channels. Clinical studies have confirmed that focused ultrasound can modulate cortical functions in the human brain, inducing plastic changes. It can alter functional connectivity in the brain and affect neurochemical substances in the deep cortex. Some studies have shown that using a navigation system to precisely target specific brain regions can safely and effectively reduce the frequency of seizures in epilepsy patients, improve symptoms of neurodegenerative diseases, alleviate neuropathic pain, and reduce depression.
Compared to existing neuromodulation techniques, FUS has several potential advantages: unlike transcranial direct current stimulation (tDCS) and transcranial magnetic stimulation (TMS), FUS can target deep brain regions with millimeter-level spatial resolution. Compared to deep brain stimulation (DBS), FUS is less invasive, avoiding surgical risks and allowing for repeated treatments. By adjusting the position or direction of the transducer, multiple brain regions such as the hippocampus, prefrontal cortex, motor cortex, caudate nucleus, and substantia nigra can be stimulated.
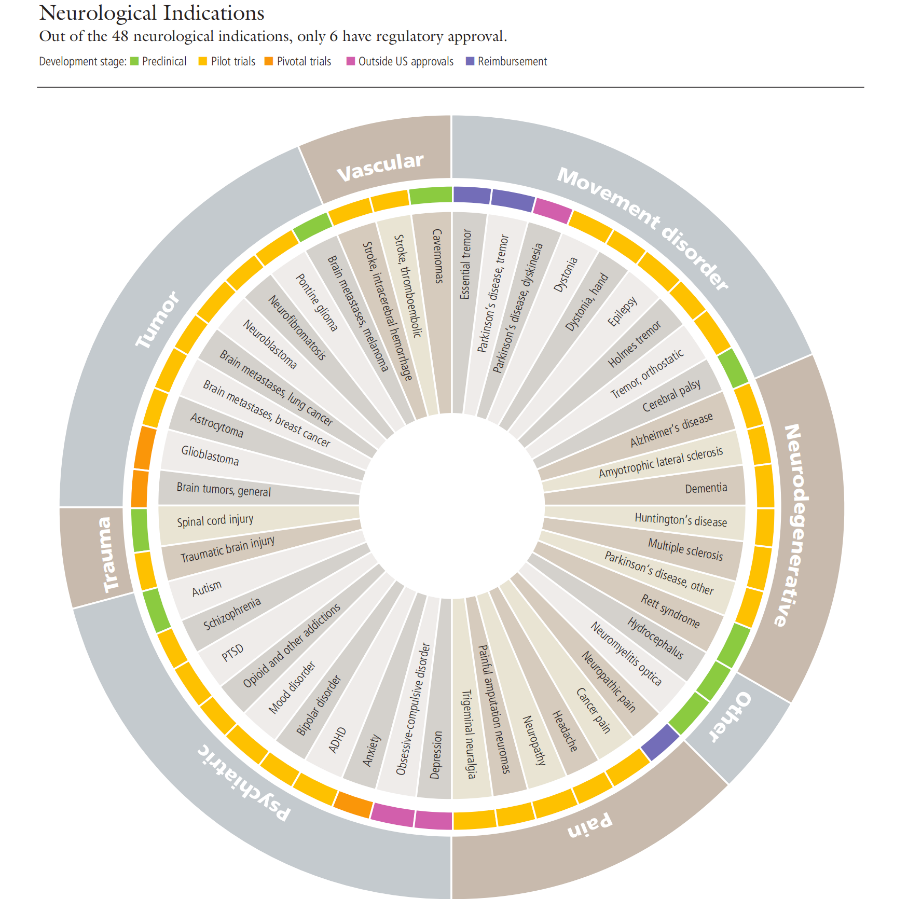
▷ Figure 4. Research status of FUS therapy for brain diseases. Source: Focused Ultrasound Foundation. “State of the Field Report 2023 – Focused Ultrasound Foundation.” Focused Ultrasound Foundation, 20 Sept. 2023, www.fusfoundation.org/the-foundation/foundation-reports/state-of-the-field-report-2023.
4. Conclusion
The role of FUS in neuroscience and clinical therapy is increasingly prominent. By precisely controlling the focused energy of sound waves, FUS not only enables precise treatment of brain lesions but also shows great potential in neuromodulation and drug delivery. Its characteristics include high localization accuracy and relative non-invasiveness.
As of 2022, the FUS field has received $3.14 billion in R&D investments from government and industry, with 337 therapies approved by 39 regulatory agencies targeting 32 indications and treating a total of 565,210 cases. Currently, dozens of therapies are still under active development.
However, the development of FUS still faces a series of technical and clinical challenges. High-intensity FUS thermal ablation therapy is currently inefficient for large lesions and surrounding brain areas, and its application is limited in patients with lower skull density. Additionally, for lesions near the skull base, the surrounding sensitive neurovascular structures may be at risk. It is anticipated that in the coming years, optimized ultrasound focusing and correction technologies, as well as more personalized ultrasound transducer arrays, will emerge to minimize heating and expand the treatment range.
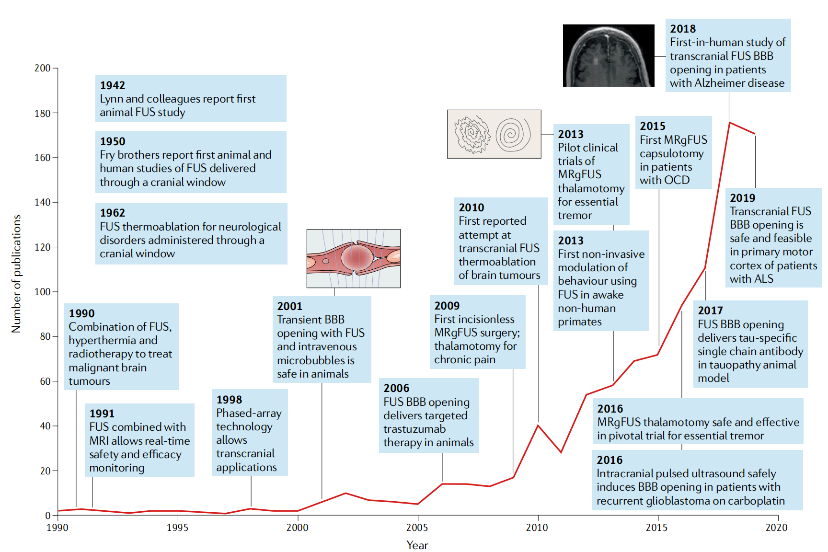
▷ Figure 5. Changes in the number of FUS-related publications over time. Source: Meng, Ying, Kullervo Hynynen, and Nir Lipsman. “Applications of focused ultrasound in the brain: from thermoablation to drug delivery.” Nature Reviews Neurology 17.1 (2021): 7-22.
Clinically, current research aims to improve the tolerability of FUS treatment, for example, by shortening surgery times and using neuroimaging-assisted devices. Additionally, exploring the application of FUS in new clinical indications, such as the treatment of brain lesions inducing epilepsy, is a future direction of development.
The future of FUS applications depends on interdisciplinary collaboration, involving joint efforts from medicine, physics, and neuroscience. Through in-depth research into its mechanisms and clinical applications, FUS holds immense potential to help us tackle some of the most challenging brain diseases faced by humanity.