Throughout human civilization, we have continuously asked: What is the nature of thought? Where does consciousness come from? How do emotions affect the body? And how is memory stored?
In 2024, ten major breakthroughs in brain science provided exhilarating new answers to these timeless questions. These advances ranged from the revolutionary discovery that language may not be an essential tool for thought to the first revelation of how stress reshapes the gut microbiota via intricate neural networks, and from mapping the first complete connectome of the fruit fly brain to observing patterns of neural activity in the human brain during its final moments. Not only do these breakthroughs expand the boundaries of our understanding of the brain, but they also profoundly transform the way we perceive ourselves.
Notably, these studies demonstrate that brain science is undergoing a paradigm shift. We no longer regard the brain as an isolated organ but as an integral part of a complex body–environment network. We are no longer limited to the electrical activities of neurons; recent findings reveal that non-neuronal cells also possess learning and memory capabilities. Moreover, we no longer view consciousness as a binary state but rather as a phenomenon with rich hierarchical complexity. These cognitive innovations are reshaping our understanding of the mind, consciousness, and human nature.
In today’s era of rapid advancements in artificial intelligence, these discoveries offer profound insights. They reveal that the working principles of the human brain are far more intricate than we had imagined. By decoding the mysteries of natural intelligence, we can not only improve the treatment of neuropsychiatric disorders but also pave the way for entirely new approaches in developing next-generation artificial intelligence. Let us step into the forefront of brain science research in 2024 and explore these groundbreaking discoveries that are transforming our understanding.
10. DNA-Sensing Receptor TLR9 Pathway Forms Memory Assemblies
Reference: Jovasevic V, Wood EM, Cicvaric A, Zhang H, et al. Formation of memory assemblies through the DNA-sensing TLR9 pathway. Nature, 2024. doi:10.1038/s41586-024-07220-7
The neurobiological mechanisms underlying memory formation have long been a central focus of neuroscience research. Although it is known that hippocampal neurons undergo molecular and cellular adaptations during information encoding, the specific signaling pathways that dictate the recruitment of certain neurons into memory circuits remain not yet fully understood. Could DNA damage and repair play a critical role in this process?
Recently, Nature published a study led by Vladimir Jovasevic and his team at the Albert Einstein College of Medicine, which investigated the key role of the DNA-sensing receptor TLR9 in memory formation. They discovered that, following several hours of learning in mice, a subset of excitatory neurons in the hippocampal CA1 region exhibited double-strand DNA breaks, nuclear envelope rupture, and the release of histone and DNA fragments. These events activated the TLR9 signaling pathway, thereby promoting DNA damage repair and the formation of memory assemblies. Moreover, by selectively knocking down the TLR9 gene in neurons, the researchers observed impaired memory function in mice, further confirming the crucial role of DNA damage repair in memory formation
Academic Impact:
(1)Theoretical Innovation: Reveals the central role of DNA damage and repair in memory formation, thereby broadening our understanding of memory mechanisms and neural plasticity.
(2)Paradigm Shift: Emphasizes the significance of atypical cellular processes, such as DNA damage repair, in neural function, thus fostering interdisciplinary research.
(3)Application Potential: Provides new evidence for developing cognitive enhancement strategies and treatments for neurodegenerative diseases based on DNA repair mechanisms.
09. Stress-Sensitive Neural Circuits Alter the Gut Microbiome via Duodenal Glands
Reference: Chang, C. et al. Stress-sensitive neural circuits change the gut microbiome via duodenal glands. Cell, 187, 1–18 (2024). doi:10.1016/j.cell.2024.07.019
The detrimental effects of stress on the gut microbiota and the consequent immune dysregulation have been widely observed, yet the neural regulatory mechanisms underlying these changes remain unclear. How does neural regulation influence the gut microbiome in response to stress?
A study published in Cell by a U.S. research team revealed that Brunner’s glands in the duodenum serve as a critical link between the brain and the gut microbiome. The researchers found that the central region of the amygdala in the forebrain is connected to Brunner’s glands via a complex vagal nerve pathway. When the vagus nerve is activated, these glands secrete specific substances that promote the enrichment of Lactobacillus in the gut. Under chronic stress, however, the activity of the amygdala is suppressed and Brunner’s gland function declines, leading to a significant reduction in Lactobacillus levels and weakened host resistance to pathogens. Moreover, optogenetic activation of either the amygdala or the vagus nerve can reverse the effects of stress by restoring Lactobacillus abundance and enhancing immune defense. Further experiments demonstrated that mice with a genetic knockout of Brunner’s glands exhibit microbial imbalance and increased susceptibility to infection, even in the absence of stress, directly confirming the central role of these glands in microbiome regulation. This discovery not only elucidates the neurobiological basis of the “stress–brain–microbiome” axis but also offers novel insights for treating stress-related conditions such as irritable bowel syndrome and autoimmune diseases—by targeting neural pathways or Brunner’s gland function to restore gut microecological balance and enhance host defense.
Academic Impact:
(1)Theoretical Breakthrough: First elucidation of the complete pathway by which stress regulates the microbiome via the brain–vagus nerve–Brunner’s gland axis, providing a new paradigm for gut–brain axis research.
(2)Clinical Value: Identifies potential therapeutic targets—such as Brunner’s glands or vagus nerve stimulation—for the treatment of stress-related disorders like irritable bowel syndrome and autoimmune diseases.
(3)Technical Implications: Supports the development of neural modulation or microbiome intervention strategies to enhance host immune defense.
▷ Source: Cell Cover: Focus on Microbes. Source: Cell
08. Language is Primarily a Tool for Communication, Not Thought
Language is widely regarded as a defining feature of humanity, yet its core function has long been a subject of debate—is it primarily meant to serve thought, or is it specialized for communication? In other words, is the essential role of language to facilitate exchange rather than to drive internal cognition?
A team from the Massachusetts Institute of Technology published a study in Nature revealing the true nature of language through neuroimaging and cognitive experiments. The researchers employed functional magnetic resonance imaging (fMRI) to monitor brain activity in subjects engaged in language tasks (such as reading) and non-language tasks (such as spatial reasoning). They observed that language processing regions (for example, Broca’s area and Wernicke’s area) were significantly activated only during language-related tasks and remained inactive during non-linguistic cognitive tasks. Further analysis indicated that activity in these language areas bore no direct relationship to abstract thought processes, such as mathematical reasoning or logical judgment. The study also highlighted that the structural features of language (including syntactic efficiency and lexical ambiguity tolerance) are optimized for information transmission rather than for enhancing internal cognition. For instance, sign language users retain full logical reasoning abilities even when their language functions are impaired, supporting the view that thought can exist independently of language.
Academic Impact:
(1)Theoretical Innovation: Challenges the traditional paradigm that “language is the medium of thought,” proposing instead that language is merely an external tool for expressing thought rather than its intrinsic driver.
(2)Interdisciplinary Implications: Provides new perspectives for artificial intelligence research, such as deconstructing design by separating language models from reasoning modules.
(3)Clinical Significance: Offers a fresh interpretation of the cognitive preservation observed in patients with language disorders (e.g., aphasia), thereby informing the optimization of rehabilitation strategies.
07. Adult Fruit Fly Brain Connectome
Reference: Winding, M. et al. Neuronal wiring diagram of an adult brain. Nature, 630, 118–126 (2024). doi:10.1038/s41586-024-07558-y
Although neuroscience has extensively investigated connectivity within localized brain regions, a global connectome has remained elusive. How, then, does a complete connectome advance our understanding of brain function and disease mechanisms?
In a study published in Nature, a team from the National Institutes of Health and Princeton University used a combination of electron microscopy imaging and automated algorithms to generate the first whole-brain connectome of an adult fruit fly, encompassing nearly 140,000 neurons and 5*107 precisely mapped synapses. This connectome not only identifies neuronal types and the distribution of neurotransmitters (such as dopamine and serotonin) but also reveals complex signal transmission pathways between brain regions. For example, it illustrates how photic signals are relayed through multiple synapses to the motor centers. Furthermore, the researchers developed an open-source platform that supports interactive data analysis and cross-species integration. This achievement provides a structural framework for elucidating the neural basis of behavioral regulation—such as learning and decision-making—and offers a novel tool for exploring connectivity abnormalities in human neurodegenerative diseases.
Academic Impact:
(1)Scientific Breakthrough: The first complete whole-brain connectome bridges the “missing link” between synaptic-level studies and behavioral research.
(2)Technological Innovation: The automated imaging and computational pipeline lays the methodological groundwork for mapping more complex brains, such as those of mice and humans.
(3)Medical Application: Comparing connectivity differences between healthy and diseased models aids research into the mechanisms underlying neurodegenerative disorders like Alzheimer’s and Parkinson’s disease.
(4)Resource Sharing: The open-source data platform fosters global collaboration, accelerating the translation of brain science into precision medicine and brain-inspired intelligence.
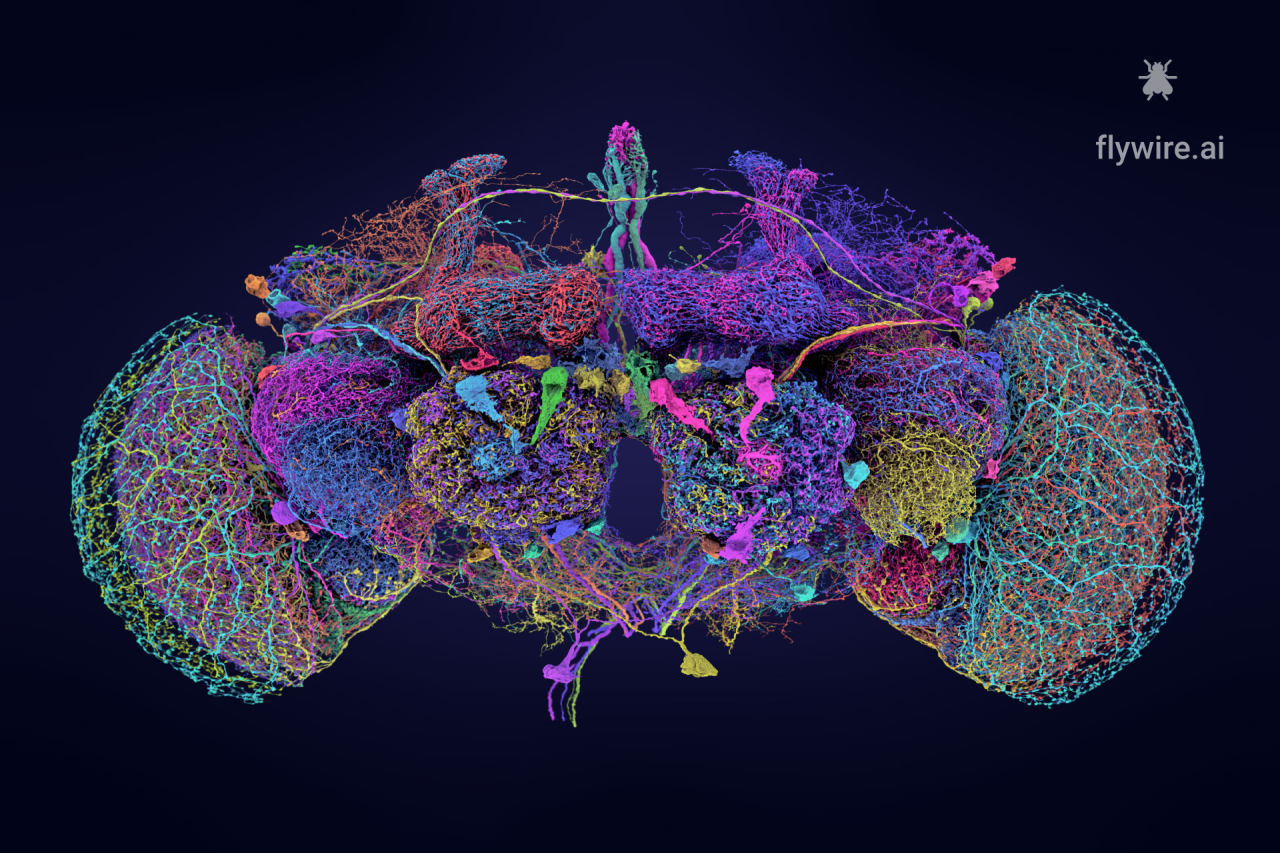
▷ Adult fruit fly brain connectome. Source: flywire.ai
06. Neuronal Dynamics Regulate Cerebrospinal Fluid Perfusion and Brain Clearance
Reference: Kipnis, J. et al. Neuronal dynamics direct cerebrospinal fluid perfusion and brain clearance. Nature, 627, 122–129 (2024). doi:10.1038/s41586-024-07108-6
The accumulation of metabolic waste in the brain is a key trigger for neurodegenerative diseases; however, the mechanisms underlying its clearance have long remained unclear. Kipnis and colleagues set out to answer a fundamental question: How does the brain clear metabolic waste?
A study by a team from Washington University School of Medicine, published in Nature, revealed that neuronal activity drives cerebrospinal fluid (CSF) flow via synchronized action potentials. The researchers found that coordinated discharges within neuronal networks generate high-amplitude, rhythmic ionic waves. These waves, in turn, induce self-sustained oscillations in the interstitial fluid (ISF). Such ionic waves enhance the efficiency of the glymphatic system, thereby promoting CSF perfusion into the brain parenchyma and facilitating the clearance of metabolic waste (such as aberrant proteins). Experimental results demonstrated that chemogenetic inhibition of these high-energy ionic waves significantly reduces both CSF flow and waste clearance efficiency, whereas optogenetic stimulation, which induces transcranial brain waves, markedly enhances the CSF–ISF exchange rate. Furthermore, the study found that high-amplitude brain waves during sleep may serve as a key regulatory factor in clearance efficiency.
Academic Impact:
(1)Mechanistic Insight: This study is the first to establish a direct link between neuronal activity and waste clearance, challenging the traditional “passive clearance” theory.
(2)Disease Intervention: It suggests novel therapeutic strategies for conditions such as Alzheimer’s disease—for example, by enhancing sleep-related brain waves to boost clearance efficiency.
(3)Technological Applications: Optogenetic modulation of brain waves may emerge as an innovative treatment for neurodegenerative diseases.

▷ Differences in γ rhythms between healthy individuals and Alzheimer’s disease patients. Source: https://brainhealth.org.uk/?p=624
05. Massed-Spaced Learning Effect in Non-Neural Human Cells
Reference: Kukushkin, N. V. et al. The massed-spaced learning effect in non-neural human cells. Nat. Commun. 15, 9635 (2024). doi:10.1038/s41467-024-53922-x
Memory formation has long been considered an exclusive function of the brain, relying on synaptic plasticity and neuronal circuits. But have you ever wondered whether non-neuronal cells might possess learning and memory capabilities similar to those of the nervous system?
A team from New York University has, for the first time, revealed the “Massed-Spaced Learning Effect” in non-neuronal cells (such as kidney cells) in a study published in Nature Communications. This effect refers to the phenomenon in which repeated stimulation delivered at intervals enhances learning and memory outcomes. The researchers simulated a learning process using pulsed stimulation with forskolin and phorbol ester and found that spaced stimulation (four pulses delivered at 10-minute intervals) resulted in luciferase expression levels that were 1.4 times higher and persisted for over 24 hours compared to massed stimulation. This process depended on the activation of key memory molecules, ERK and CREB. Since this effect is observed in both neural and non-neural cells, it suggests that memory capabilities may emerge from the universal dynamics of cellular signaling networks rather than being an exclusive function of the nervous system.
Academic Impact:
(1)Theoretical Breakthrough: Expands the biological boundaries of memory by proposing the “cellular-level memory” hypothesis.
(2)Medical Potential: Offers novel, non-neural targeted therapeutic approaches for memory-related disorders, such as Alzheimer’s disease.
(3)Technological Applications: Establishes a cellular memory model to aid in drug development and toxicity assessments.
▷ Source: MIT.edu
04. Reconstructing the Human Cerebral Cortex at Nanoscale Resolution
Reference: Shapson-Coe, A. et al. A petavoxel fragment of human cerebral cortex reconstructed at nanoscale resolution. Science, 384, eadk4858 (2024). doi:10.1126/science.adk4858
The intricate microarchitecture and synaptic connectivity of the human cerebral cortex are key to understanding cognitive function. However, due to the resolution limits and data scale constraints of traditional imaging techniques, the ultrastructural details have long remained largely unrevealed. Can nanoscale imaging techniques, therefore, unravel the ultrastructure and synaptic connectivity of the human cerebral cortex?
In a collaborative study between Google and Harvard University’s Lichtman Laboratory, published in Science, researchers employed serial-section electron microscopy (with a 4 nm resolution) to image a 1-cubic-millimeter sample of the human temporal cortex obtained during epilepsy surgery. Using automated computational methods, they reconstructed the largest brain ultrastructure dataset to date (H01, 1.4 PB), which comprises approximately 57,000 cells, 230 millimeters of vasculature, and 150 million synapses—yielding, for the first time, a comprehensive cross-cortical synaptic connectivity map. The study revealed a glial cell-to-neuron ratio of 2:1, with oligodendrocytes representing the highest proportion. Moreover, deep-layer excitatory neurons could be classified into functional subtypes based on dendritic orientation. In addition, each neuron received thousands of weak synaptic connections alongside a few “potent inputs” (with a single axon forming up to 50 synapses), suggesting a hierarchical amplification mechanism in neural information processing. The research team overcame challenges such as the rapid degradation of brain tissue samples and the enormous volume of imaging data, thereby providing the first nanoscale “structure-function” map for neural circuits. All related data have been made openly available.
Academic Impact:
(1)Theoretical Breakthrough: Challenges the macroscopic limitations of traditional brain mapping by revealing the heterogeneity of synaptic connectivity, thereby providing structural support for neural coding theories.
(2)Technological Innovation: The integration of 4 nm resolution serial-section electron microscopy with AI-driven automated annotation (Serial EM) sets a new benchmark in large-scale biological imaging.
(3)Data Sharing: The open-source H01 dataset will accelerate research into brain disorder mechanisms (such as epilepsy and schizophrenia) and the development of brain-inspired computational models.
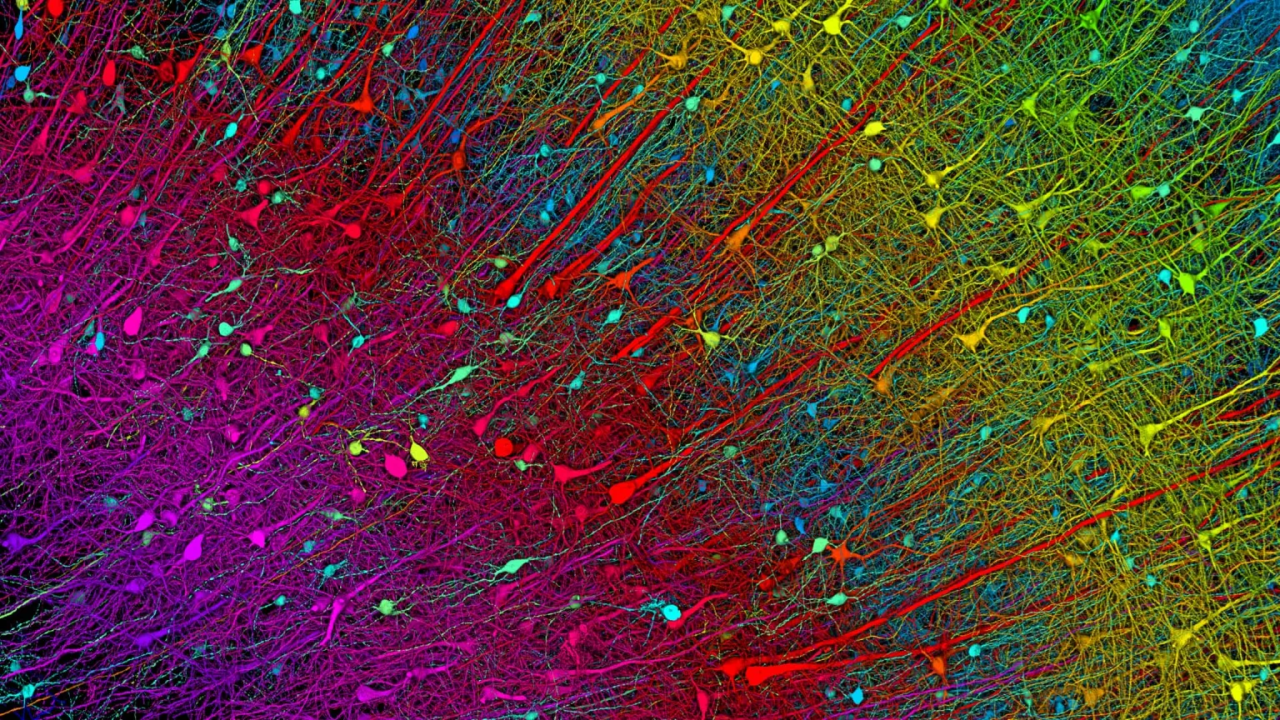
▷ Excitatory neuron staining (six layers). Source: Harvard University, Google Research, and the Lichtman Laboratory
03. Psilocybin Desynchronizes the Human Brain
Reference: Dosenbach, N. U. F. et al. Psilocybin desynchronizes the human brain. Nature 630, 1020–1028 (2024). doi:10.1038/s41586-024-07624-5
Psilocybin, a psychedelic agent with rapid antidepressant potential, has a mechanism for promoting neural plasticity through the disruption of brain network synchrony that remains unclear. How does it modulate brain network synchrony to yield both rapid and enduring therapeutic effects?
In a study published in Nature, a team from Washington University School of Medicine in St. Louis employed longitudinal precision functional mapping techniques—where healthy subjects underwent an average of 18 MRI scans—to systematically track the acute and long-term effects on brain functional connectivity of a high dose of psilocybin (25 mg) compared with the control drug methylphenidate (40 mg). The study found that psilocybin triggers a dramatic, widespread desynchronization across the brain. During the acute phase, the functional connectivity between cortical and subcortical regions was disrupted to more than three times the extent observed with the control drug. This disruption was characterized by decreased intra-network correlations and weakened inter-network anticorrelations. Notably, the collapse of synchrony was most significant in the default mode network (DMN)—especially in nodes connected to the anterior hippocampus—and was directly linked to subjects’ subjective psychedelic experiences, such as distortions in spatial-temporal perception and depersonalization. Importantly, the reduced functional connectivity between the anterior hippocampus and the DMN persisted for several weeks, paralleling the enhanced neural plasticity observed in animal models. This finding offers a potential neural mechanism underlying the therapeutic effects of psilocybin.
Academic Impact:
(1)Mechanistic Insight: Proposes a “desynchronization-resynchronization” model that elucidates how psychedelics facilitate brain remodeling by disrupting entrenched network states.
(2)Clinical Translation: Suggests that the connectivity strength between the DMN and the anterior hippocampus may serve as a biomarker and therapeutic target for disorders such as depression and addiction.
(3)Methodological Innovation: Demonstrates that personalized longitudinal functional mapping techniques provide a high temporal resolution framework for evaluating and developing psychopharmacological treatments.

▷ Source: SciTechDaily.com
02. Surge in Neurophysiological Coupling and Connectivity of Gamma Oscillations in the Dying Human Brain
Reference: Borjigin, J. et al. Surge of neurophysiological coupling and connectivity of gamma oscillations in the dying human brain. Proc. Natl. Acad. Sci. U.S.A. 120, e2216268120 (2023). doi:10.1073/pnas.2216268120
Traditional views have held that brain activity is profoundly suppressed during cardiac arrest; however, new insights have emerged regarding changes in neural activity in the dying human brain. In a study published in the Proceedings of the National Academy of Sciences (PNAS), a team led by Jimo Borjigin at the University of Michigan analyzed electroencephalogram (EEG) data from four cardiac arrest patients to reveal the complexity of neurophysiological activity at the end of life.
The study found that following the cessation of respiratory support, two patients exhibited a significant enhancement in gamma-band (25–150 Hz) EEG power—up to three times their baseline levels. This surge was accompanied by increased cross-frequency coupling (for example, enhanced synchrony between gamma and beta oscillations) and a marked rise in interhemispheric functional connectivity. Such activity was predominantly localized in the temporo-parieto-occipital (TPO) junction, a region closely associated with consciousness integration, visuospatial perception, and dream generation. Furthermore, the enhancement of gamma oscillations coincided with deteriorating cardiac function, suggesting that hypoxia may drive the abnormal activation of neural networks. Notably, this activity pattern resembles the EEG features observed in healthy individuals during recollection or dreaming, potentially offering a neural mechanism for the “life review” phenomenon often reported in near-death experiences.
Academic Impact:
(1)Theoretical Innovation: This study challenges the traditional hypothesis of “brain silence” at the end of life by proposing that hypoxia may trigger a compensatory state of heightened neural activity.
(2)Clinical Implications: It opens new avenues for investigating the neural basis of near-death experiences and may drive the development of technologies for detecting covert consciousness.
(3)Technological Breakthrough: The application of high-density EEG and functional connectivity analysis provides a methodological paradigm for studying the brain under extreme physiological conditions.
01. Neuroanatomical Changes During Human Pregnancy
Reference: Pritschet L, Taylor CM, Cossio D, et al. Neuroanatomical changes observed over the course of a human pregnancy. Nat. Neurosci. 27, 2253–2260 (2024). doi:10.1038/s41593-024-01741-0
The dramatic hormonal and physiological upheavals triggered by pregnancy have long been understudied with respect to their impact on the maternal brain. This raises an intriguing question: How do the neuroanatomical structures of the human brain dynamically change during pregnancy?
A team from the University of California, Santa Barbara, conducted a groundbreaking longitudinal study using high-resolution MRI to track structural changes in a woman’s brain from three weeks before pregnancy to two years postpartum (a total of 26 scans). The study found that during pregnancy the brain undergoes significant remodeling. This remodeling is characterized by widespread reductions in gray matter volume and cortical thickness—affecting up to 80% of cortical regions (for example, the prefrontal and posterior parietal cortices)—as well as enhanced integrity of white matter microstructure and increases in both ventricular and cerebrospinal fluid (CSF) volumes. Notably, some of the reductions in gray matter did not fully recover even two years postpartum, whereas white matter alterations gradually returned to baseline following delivery. Further analysis revealed that these structural changes were closely associated with fluctuations in hormone levels, including estradiol and progesterone, suggesting that they may represent a “neural resource reallocation” in the maternal brain to meet the demands of caregiving. These findings were published in Nature Neuroscience, and all imaging and hormonal datasets have been made openly available.
Academic Impact:
(1)Theoretical Innovation: Challenges the traditional notion of structural stability in the adult brain by demonstrating that pregnancy can drive large-scale neuroplasticity.
(2)Clinical Value: Provides potential neurobiological markers for perinatal depression and cognitive impairments, thereby facilitating the development of early intervention strategies.
(3)Resource Sharing: The first comprehensive dataset of brain imaging throughout pregnancy will accelerate research into the neuroprotective mechanisms underlying maternal brain adaptation.