Throughout the history of medical development, we have persistently asked: How can we help paralyzed patients regain the ability to stand? How can we prevent the gradual fading of memories in dementia? And for those deeply affected by depression, how can we restore their hope for life? In 2024, ten major breakthroughs in the field of neuropsychiatric disorders have provided transformative insights into these core issues affecting human health.
These breakthroughs have not only expanded the boundaries of our understanding of neurological diseases but have also opened entirely new avenues for clinical treatment. They range from the groundbreaking discovery that deep brain stimulation of the hypothalamus can promote motor function recovery following spinal cord injury, to the first revelation of dual pathological features in the salience network of patients with depression. Other advances include the construction of the first multimodal cellular atlas for Alzheimer’s disease (AD) and the finding that the novel coronavirus can directly attack dopaminergic neurons.
Excitingly, these studies demonstrate that the field of neuromedicine is undergoing a profound paradigm shift. We are no longer confined to traditional single-drug therapies but are beginning to integrate diverse approaches. We are moving beyond mere symptom management to delve into the underlying pathological mechanisms in search of fundamental solutions. Most importantly, the principles of preventive medicine—ranging from a lifespan approach to dementia prevention to early screening using blood-based biomarkers—are reshaping our models for disease diagnosis and treatment. Join us at the forefront of neuropsychiatric research in 2024 as we explore these groundbreaking discoveries that bring new hope to medicine.
10. Hypothalamic Deep Brain Stimulation Promotes Gait Recovery Following Spinal Cord Injury
Reference: Cho N, Squair JW, Aureli V, et al. Hypothalamic deep brain stimulation augments walking after spinal cord injury. Nat Med. 2024. doi:10.1038/s41591-024-03306-x
Spinal cord injury (SCI) disrupts the neural pathways between the brain and the spinal cord. While traditional interventions, such as spinal cord stimulation, can partially alleviate symptoms, achieving long-lasting functional rehabilitation has remained challenging. Can neuromodulation technologies enable the recovery of motor functions following SCI?
In a study published in Nature Medicine by a team from the École Polytechnique Fédérale de Lausanne (EPFL) and the Lausanne University Hospital (CHUV), a novel mechanism was revealed showing that deep brain stimulation (DBS) promotes neural plasticity by targeting an unconventional motor regulatory region—the lateral hypothalamus (LH). The study found that activation of glutamatergic neurons in the LH drives the formation of compensatory connections between the ventral gigantocellular nucleus (vGi) in the brainstem and the remaining spinal pathways, resulting in improved gait function in rodent models. In a clinical trial involving two patients with chronic, incomplete SCI, LH-DBS significantly increased walking speed and endurance while also inducing long-term adaptive restructuring of neural networks. Notably, even after stimulation was turned off, motor function continued to improve.
This discovery overturns the traditional notion that motor functions are solely driven by corticospinal pathways and proves that the hypothalamus can integrate neural networks across multiple levels to achieve functional compensation, offering a new paradigm for spinal cord injury treatment.
Academic Impact:
(1)Theoretical Innovation: Overturning traditional localization of motor control regions, this finding reveals that the hypothalamus drives neural remodeling through compensatory pathways between the brainstem and spinal cord.
(2)Clinical Translation: DBS combined with rehabilitative training breaks the bottleneck in chronic spinal cord injury functional recovery, transitioning from mere symptom relief to neural repair.
(3)Technological Benchmark: Cross-species whole-brain spatiotemporal mapping provides standardized pathways for target screening and intervention strategies in complex neurological diseases.
09. Expansion of the Frontostriatal Salience Network in Individuals with Depression
Reference: Lynch CJ, Elbau IG, Ng TH, et al. Frontostriatal salience network expansion in individuals with depression. Nature. 2024;633(8030):624-633. doi:10.1038/s41586-024-07805-2
Research into the neurobiological mechanisms of depression has long been constrained by a lack of biomarkers. Is there a brain network biomarker for depression that is both stable and dynamic?
A research team from Weill Cornell Medical College, in a study published in Nature, used intensive longitudinal brain imaging (up to 62 scans per individual) to reveal dual pathological features of the salience network. At the structural level, the cortical coverage of the network in patients was nearly twice that observed in healthy individuals, and this expansion was already evident before symptom onset (e.g., in prepubertal children), suggesting its potential as a neurodevelopmental risk marker. At the functional level, fluctuations in the strength of key connections—such as those between the nucleus accumbens and the anterior cingulate cortex—were dynamically correlated with core symptoms like anhedonia and anxiety and were predictive of future symptom worsening. The study further found that the salience network, by encroaching upon the spatial resources of the default mode network and the frontoparietal network, disrupted the coordinated interplay among multiple networks and led to impairments in cognitive–affective integration.
This dual “structural stability–functional dynamism” model transcends the traditional one-dimensional view of biomarkers, offering a novel paradigm for early warning (based on structural features) and personalized treatment (by tracking functional fluctuations) in depression.
Academic Impact:
(1)Diagnostic Innovation: Enhanced diagnostic objectivity based on brain network features (accuracy rate of 78.4%).
(2)Treatment Optimization: Dynamic connectivity features guiding precise neuromodulation interventions.
(3)Paradigm Shift: Establishment of new standards for longitudinal multimodal brain imaging studies.
(4)Early Warning Breakthrough: Identification of childhood network biomarkers to pinpoint optimal prevention windows.
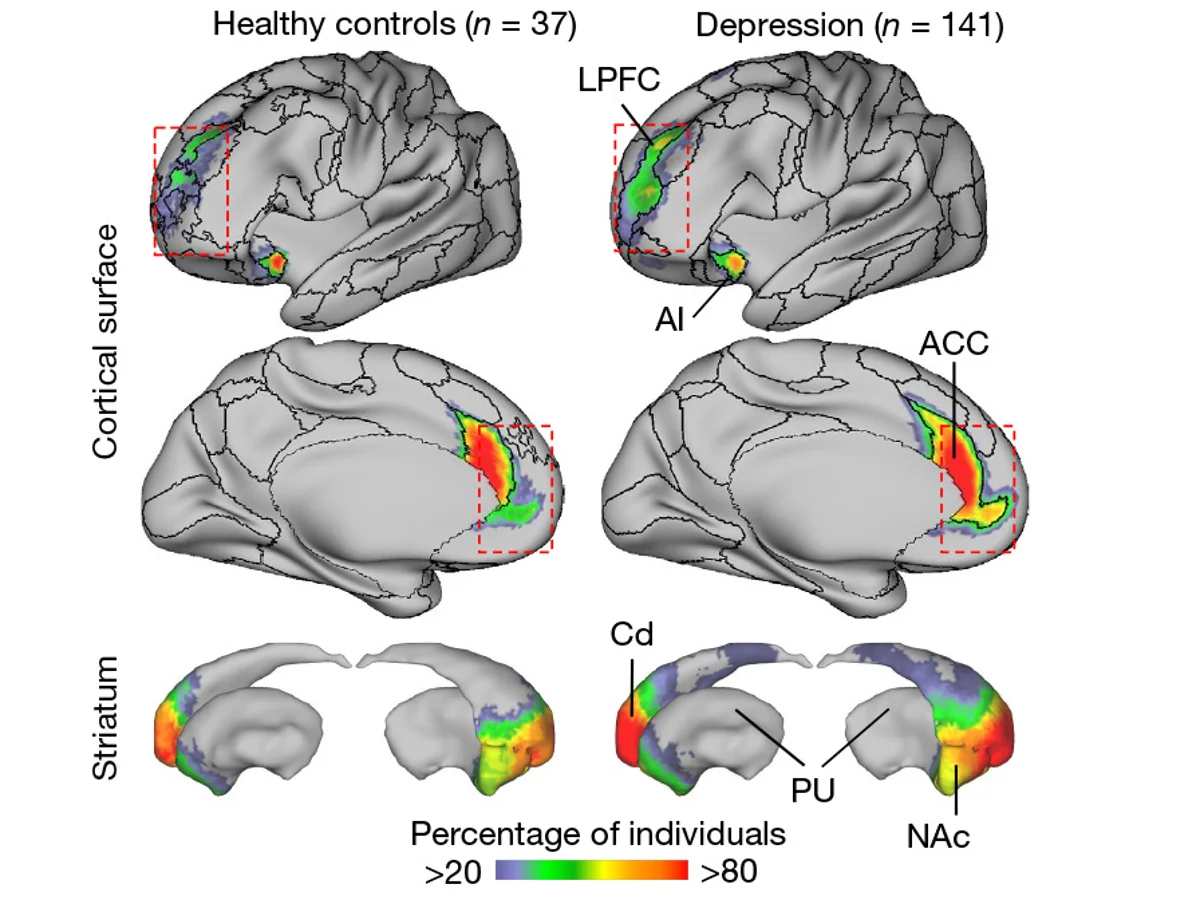
▷ Source: Nature
08. Integrated Multimodal Cell Atlas of Alzheimer’s Disease
Reference: Gabitto MI, Travaglini KJ, et al. Integrated multimodal cell atlas of Alzheimer’s disease. Nat Neurosci. 2024;27(7):1-15. doi:10.1038/s41593-024-01774-5
The pathological progression of Alzheimer’s disease (AD) involves complex cellular dynamics, yet its spatiotemporal heterogeneity has long remained uncharacterized. Can specific alterations in brain cells precipitate the worsening of AD?
A collaborative team from the National Institutes of Health (NIH) and the Allen Institute, in a study published in Nature Neuroscience, integrated multi-omics, spatial genomics, and the BRAIN Initiative reference atlas to construct the first multimodal cell atlas of AD. The study analyzed middle temporal gyrus samples from 84 AD patients (mean age at death: 88 years) and, using a disease pseudo-progression scoring system, uncovered a “two-stage model” of AD progression. In the first stage, pathology accumulates gradually, accompanied by the activation of inflammatory cells (such as microglia) and partial loss of inhibitory neurons, while intrinsic repair mechanisms (e.g., myelin regeneration) are initiated. In the subsequent stage, there is an exponential escalation of pathology with massive loss of critical neurons (such as excitatory neurons), culminating in a complete collapse of cognitive function.
Cross-cohort validation confirmed that this “two-stage model” applies broadly to amyloid deposition, tau pathology, and cognitive decline, indicating that the coordinated degeneration of neuronal and non-neuronal cells is the core mechanism underlying AD progression.
Academic Impact:
(1)Pathological Mechanism Breakthrough: For the first time, a systematic delineation of cell type–specific responses in AD provides a precise atlas for targeted interventions.
(2)Diagnostic Stratification Tool: The disease pseudo-progression scoring system optimizes patient staging and guides the selection of individualized treatment windows.
(3)Therapeutic Target Discovery: Molecular pathways linking early inflammatory microglial activation with late-stage neuronal loss may serve as focal points for novel drug development.
(4)Methodological Paradigm Shift: The multimodal integrative strategy offers a template for research into other neurodegenerative diseases.
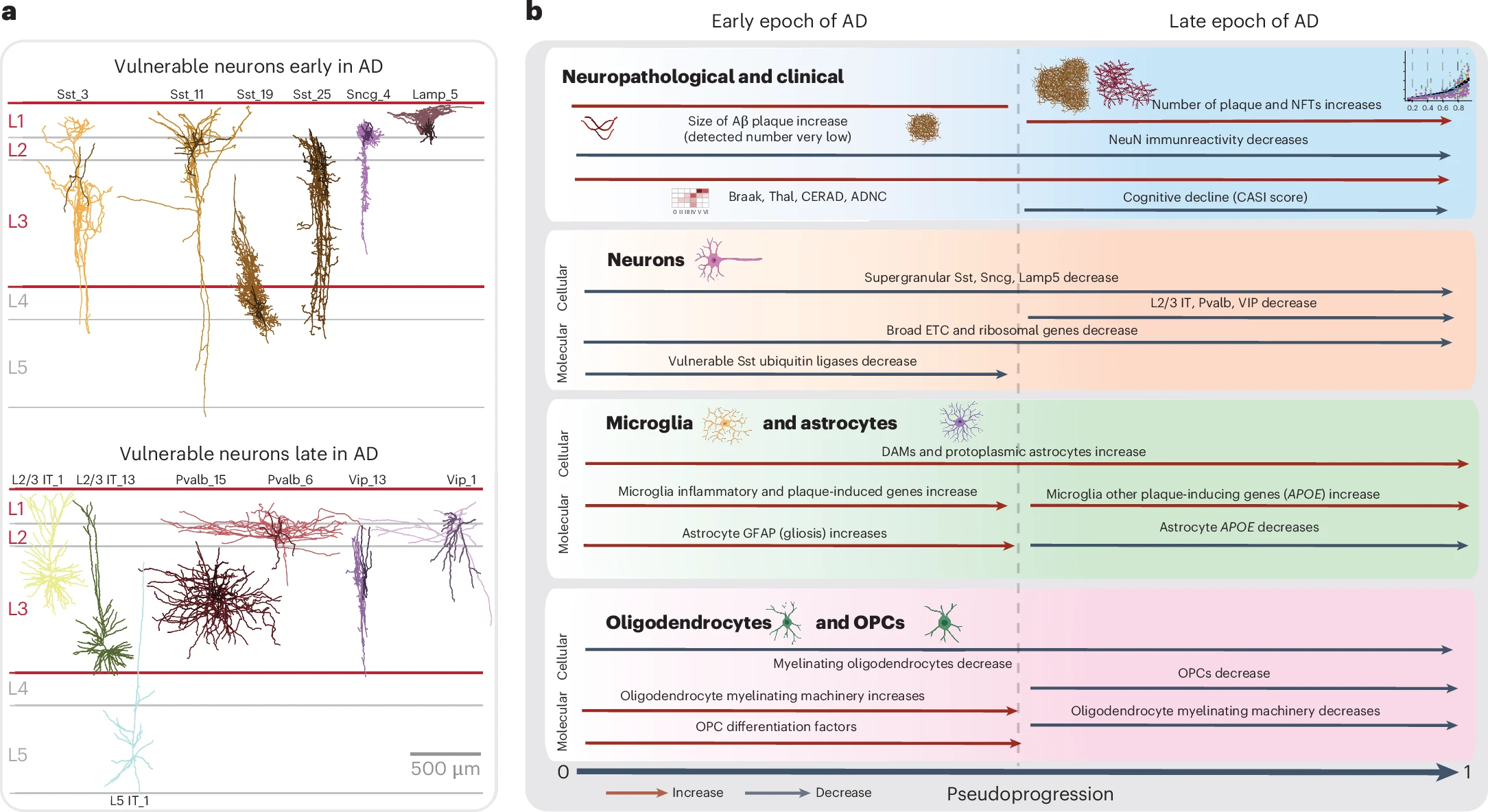
▷ Source: Nature
07. Restoring Hippocampal Glucose Metabolism Rescues Cognitive Impairment in Alzheimer’s Disease
Reference: Minhas PS, Jones JR, Latif-Hernandez A, et al. Restoring hippocampal glucose metabolism rescues cognition across Alzheimer’s disease pathologies. Science. 2024;385(6711):eabm6131. doi:10.1126/science.abm6131
Disruptions in brain glucose metabolism in Alzheimer’s disease (AD) have long been considered secondary phenomena, although their causal role remains unclear. Could correcting these metabolic deficits reverse the cognitive impairment observed in AD? What is the underlying mechanism?
A research team from the University of Pennsylvania, in a study published in Science, revealed that aberrant activation of indoleamine 2,3-dioxygenase 1 (IDO1) in astrocytes is the key driver of metabolic dysfunction in AD. The study found that amyloid‐β (Aβ) and tau oligomers activate IDO1, which converts tryptophan (TRP) into kynurenine (KYN). Kynurenine, in turn, suppresses the expression of critical glycolytic enzymes via aryl hydrocarbon receptor (AhR) signaling, resulting in reduced lactate production.
In AD mouse models, inhibition of IDO1 restored hippocampal glucose metabolism, rescuing long-term potentiation (LTP) and memory function. Furthermore, using a co-culture model of astrocytes and neurons derived from AD patients, researchers confirmed that an IDO1 inhibitor enhanced lactate secretion and improved neuronal metabolic uptake, indicating its ability to directly repair neuroglial metabolic coupling.
Academic Impact:
(1)Mechanistic Breakthrough: For the first time, the “IDO1-KYN-AhR” axis has been established as the central pathway driving metabolic dysfunction in AD, challenging the traditional amyloid hypothesis.
(2)New Therapeutic Target: IDO1 inhibitors (such as Epacadostat), already evaluated in cancer clinical trials, may be rapidly repurposed for AD treatment.
(3)Metabolic Intervention Strategy: Restoration of brain lactate metabolism has been shown to rescue cognitive function, offering a novel non-antibody–based therapeutic approach for AD.
(4)Diagnostic Biomarker Potential: Cerebrospinal fluid (CSF) levels of KYN could serve as biomarkers for early metabolic dysregulation in AD.
06. Cognitive-Motor Dissociation in Disorders of Consciousness
Reference: Bodien YG, Allanson J, Cardone P, et al. Cognitive Motor Dissociation in Disorders of Consciousness. N Engl J Med. 2024;391(7):598-608. doi:10.1056/NEJMoa2400645
In patients with disorders of consciousness, some individuals—despite showing no overt behavioral responses—may still retain covert cognitive abilities, a phenomenon known as “cognitive-motor dissociation.” How can these hidden cognitive capacities be accurately identified?
An international team led by Massachusetts General Hospital conducted a 15-year multicenter study, published in The New England Journal of Medicine, that systematically assessed the consciousness states of 353 patients using functional magnetic resonance imaging (fMRI) combined with electroencephalography (EEG). The study found that among 241 patients who exhibited no visible responses, 25% demonstrated neural activity in response to commands—for example, EEG-detected activation during an “imagine clenching your fist” task—thereby confirming the presence of cognitive-motor dissociation. These patients were more commonly younger, had experienced traumatic brain injury, and had a longer disease duration. In contrast, 38% of patients who displayed overt behavioral responses exhibited even more complex covert cognitive activity. The research emphasizes that relying solely on behavioral observations can severely underestimate a patient’s level of consciousness, potentially leading to inadequate care or premature withdrawal of life support.
Academic Impact:
(1)Diagnostic Innovation: Promotes the combined use of fMRI and EEG as the gold standard for consciousness assessment, thereby reducing the risk of misdiagnosis.
(2)Clinical Decision Optimization: Facilitates the identification of patients with cognitive-motor dissociation, guiding individualized rehabilitation plans and communication strategies.
(3)Ethical Practice Challenges: Calls for a redefinition of the medical and legal standards of “consciousness” to prevent ethical controversies in life support decisions.
(4)Prognostic Evaluation Improvements: Establishes that the presence of covert cognitive abilities is linked to the potential for neural functional recovery, thereby predicting the likelihood of long-term rehabilitation.
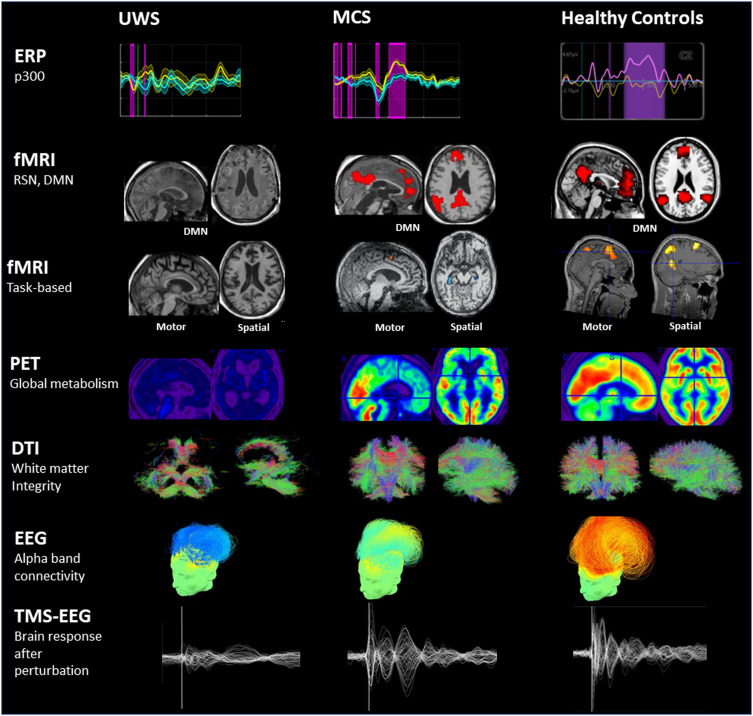
▷ Typical neuroimaging and neurophysiological findings in patients with Unresponsive Wakefulness Syndrome (UWS), Minimally Conscious State (MCS), and healthy controls. Source: ScienceDirect
05. SARS-CoV-2 Infection Causes Dopaminergic Neuron Senescence
Reference: Yang L, Kim TW, Han Y, et al. SARS-CoV-2 infection causes dopaminergic neuron senescence. Cell Stem Cell. 2024;31(2):196-211.e6. doi:10.1016/j.stem.2023.12.012
The mechanisms underlying the neurological sequelae of COVID-19 have long been unclear, hindering the development of targeted therapeutic strategies. A study published in Cell Stem Cell in 2024 addressed this question: How does SARS-CoV-2 infection contribute to long-term neurodegenerative risk?
Using a human stem cell–derived neuronal model, a team from Weill Cornell Medicine discovered that the virus invades dopaminergic neurons via the ACE2 receptor, thereby activating inflammatory and senescence pathways. This activation leads to reduced neuromelanin synthesis and decreased expression of proteins associated with motor function. The phenomenon was further confirmed in the substantia nigra of patients who died from COVID-19, where a significant loss of dopaminergic neurons was observed. Moreover, the study identified three FDA-approved drugs—Riluzole, Metformin, and Imatinib—as potential therapeutics. These drugs were shown to reverse neuronal damage by either blocking viral entry or inhibiting senescence signaling pathways.
This discovery not only elucidates the biological basis for Parkinsonism-like symptoms observed in COVID-19 patients but also, for the first time, reveals the core pathological mechanism by which SARS-CoV-2 directly attacks dopaminergic neurons and induces cellular senescence. Furthermore, it provides direct evidence supporting the repurposing of existing drugs to combat the neurological sequelae of COVID-19 and underscores the need for long-term monitoring of neurodegeneration in infected individuals.
Academic Impact:
(1)Mechanistic Breakthrough: Reveals a novel pathological pathway by which SARS-CoV-2 directly attacks dopaminergic neurons.
(2)Therapeutic Translation: Identifies three neuroprotective drugs with potential for rapid clinical translation.
(3)Clinical Early Warning: Calls for systematic monitoring of neurodegenerative disease risks, such as Parkinson’s disease.

▷ Neuronal Injury and Elevated Neuroinflammation in Brain Tissue of COVID-19 Infected Patients. Source: Danielle Beckman
04. Home-Based Transcranial Direct Current Stimulation for Major Depressive Disorder: A Fully Remote Phase II Randomized Controlled Trial
Reference: Woodham RD, Selvaraj S, Lajmi N, et al. Home-based transcranial direct current stimulation treatment for major depressive disorder: a fully remote phase 2 randomized sham-controlled trial. Nat Med. 2025;31(1):87-95. doi:10.1038/s41591-024-03305-y
Patients with depression often experience a suboptimal response to pharmacotherapy or struggle with side effects, highlighting the urgent need for accessible and well-tolerated alternative treatments. In 2024, the world’s first fully remote Phase II clinical trial of home-based transcranial direct current stimulation (tDCS) was completed. Therefore, how effective is non-invasive brain stimulation therapy in remotely alleviating major depressive disorder?
In a study published in Nature Medicine, an international, multicenter team conducted the first fully remote, double-blind, sham-controlled Phase II clinical trial (NCT05202119) to evaluate the efficacy and safety of tDCS. A total of 174 patients with major depressive disorder were randomly assigned to receive either active tDCS or sham stimulation. The results demonstrated that the active treatment group experienced a significantly greater reduction in depressive symptoms compared to the sham group. Specifically, Hamilton Rating Scale for Depression scores improved by 9.41 points in the active group versus 7.14 points in the sham group, with the therapeutic effect persisting into the open-label phase. Remote monitoring ensured high adherence (85% completion rate), and only minor adverse events—such as mild scalp irritation—were reported.
The study confirmed that by modulating prefrontal neural activity, tDCS offers a safe and convenient new option for patients who are treatment-resistant, reside in remote areas, or decline pharmacotherapy.
Academic Impact:
(1)Therapeutic Innovation: The first fully remote tDCS protocol fills a critical gap in non-pharmacological treatments.
(2)Enhanced Accessibility: Home-based treatment lowers barriers to care, benefiting patients with treatment-resistant depression and those in remote locations.
(3)Model Transformation: Remote monitoring optimizes healthcare resource allocation and advances the practice of precision psychiatry.
03. Non-Invasive Spinal Cord Electrical Stimulation for Arm and Hand Dysfunction in Chronic Tetraplegia
Reference: Moritz C, Field-Fote EC, Tefertiller C, et al. Non-invasive spinal cord electrical stimulation for arm and hand function in chronic tetraplegia: a safety and efficacy trial. Nat Med. 2024;30(5):1276-1283. doi:10.1038/s41591-024-02940-9
Patients with chronic cervical spinal cord injury (SCI) have traditionally relied on invasive treatments to address the loss of arm and hand function. However, the surgical risks and high costs associated with these approaches have limited their widespread use. Can non-invasive spinal cord electrical stimulation safely restore upper limb function in patients with chronic tetraplegia?
A team from the École Polytechnique Fédérale de Lausanne (EPFL) in Switzerland recently published a study in Nature Medicine that validated the clinical value of a non-invasive electrical stimulation technology—specifically, ARCEX therapy—through a multicenter clinical trial. This technique employs surface electrodes to target and modulate spinal cord neural circuits and is combined with rehabilitation training to promote neuroplasticity. The results demonstrated that 72% of patients with chronic tetraplegia (with injuries of at least one year) achieved statistically significant improvements in both strength and function.
This breakthrough not only circumvents the risks inherent to surgical implantation but also provides the first evidence that non-invasive electrical stimulation can induce functional reorganization by activating the intrinsic neural networks of the spinal cord, thereby offering a novel paradigm for neural repair.
Academic Impact:
(1)Therapeutic Innovation: The non-invasive design overcomes the need for surgery, broadening treatment options for patients at high risk of infection.
(2)Enhanced Accessibility: With treatment costs reduced by 40% and the availability of portable devices that support home-based rehabilitation, this approach benefits primary care settings.
(3)Mechanistic Expansion: Strategies that activate spinal neural plasticity introduce new therapeutic targets for degenerative disorders such as Parkinson’s disease.
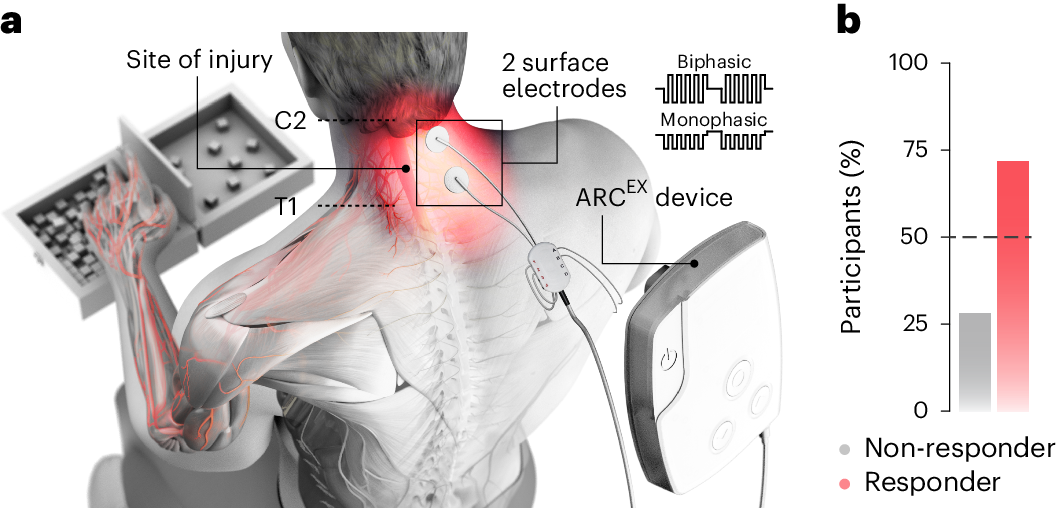
▷ Source: Nature Medicine
02. Dementia Prevention, Intervention, and Care: 2024 Report of The Lancet Standing Commission
Reference: Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet. 2024;403(10442):P2673-2736. doi:10.1016/S0140-6736(24)01296-0
Dementia may begin silently more than a decade before clinical diagnosis. So, what are the similarities and differences among interventions aimed at reducing dementia risk over the entire life course?
The 2024 report from the Lancet Dementia Prevention Commission expands the list of modifiable risk factors for dementia by including impaired vision and high LDL cholesterol, bringing the total number of modifiable risk factors to 14. Analysis of multinational cohort data confirmed that sustained interventions—from childhood (such as education and social support) to older age (such as managing vascular risk factors and protecting against brain injuries)—can significantly reduce the risk of dementia. Even among individuals with high genetic risk, such interventions can lower the likelihood of developing dementia by 40%.
Based on these findings, the report puts forward 13 global action recommendations that encompass both individual behaviors (e.g., smoking cessation and cognitive training) and policy measures (e.g., improving air quality and enhancing community support). Economic models indicate that, in the UK alone, implementing interventions (such as reducing excessive alcohol consumption and controlling hypertension) could save approximately £4 billion in healthcare and social costs.
Academic Impact:
(1)Expansion of Intervention Strategies: For the first time, interventions addressing visual impairment and high LDL cholesterol are included, shifting dementia prevention from an elderly-centric focus to a life-course approach.
(2)Economic Value: The demonstrated long-term cost-effectiveness of preventive investments encourages governments to prioritize public health policies.
(3)Personalized Prevention: Integrating genetic risk stratification with interventions targeting modifiable risk factors offers precise management pathways for high-risk populations.
(4)Global Action Framework: The 13 recommendations integrate medical and social support, calling for cross-sector collaboration to address the dementia crisis.
01. Blood Biomarkers in Primary and Secondary Prevention of Alzheimer’s Disease
Reference: Palmqvist S, Tideman P, Cullen N, et al. Blood biomarkers to detect Alzheimer disease in primary care and secondary care. JAMA. 2024;332(4):321-330. doi:10.1001/jama.2024.13855
Traditional pathological diagnosis of Alzheimer’s disease (AD) relies on invasive procedures such as cerebrospinal fluid analysis or high-cost imaging examinations, thereby limiting the accessibility of early screening. Can blood biomarkers effectively improve the diagnostic accuracy of AD in both primary and secondary prevention settings?
A team from Lund University in Sweden, in a study published in JAMA, validated the clinical diagnostic performance of a plasma-based Amyloid Probability Score 2 (APS2) and the percentage of p-tau217 through a prospective multi-cohort analysis involving 1,213 patients with cognitive symptoms. The study found that in the primary care cohort, APS2 achieved a diagnostic accuracy of 91% (AUC 0.97), significantly outperforming the clinical assessments of primary care physicians (61%). In the secondary care cohort, APS2 demonstrated similarly high accuracy (88%–91%), surpassing that of dementia specialists (73%). Notably, when used alone, the p-tau217 percentage achieved a diagnostic accuracy of 90%, which is equivalent to that of APS2, suggesting that it can serve as an independent biomarker to simplify the testing process. Furthermore, the study confirmed that 50% of patients with cognitive symptoms exhibit AD pathology, and that blood tests can accurately identify these individuals, thereby reducing reliance on traditional methods.
Academic Impact:
(1)Diagnostic Innovation: Blood testing achieves an accuracy exceeding 90%, providing a universal tool for early AD screening.
(2)Process Optimization: Simplifies the primary care pathway and reduces the need for specialist referrals.
(3)Resource Replacement: Serves as an alternative to high-cost examinations (such as PET), potentially saving 30% in annual healthcare expenditures.
(4)Guideline Revision: Promotes the integration of blood biomarkers into clinical guidelines, thereby advancing the early treatment window.
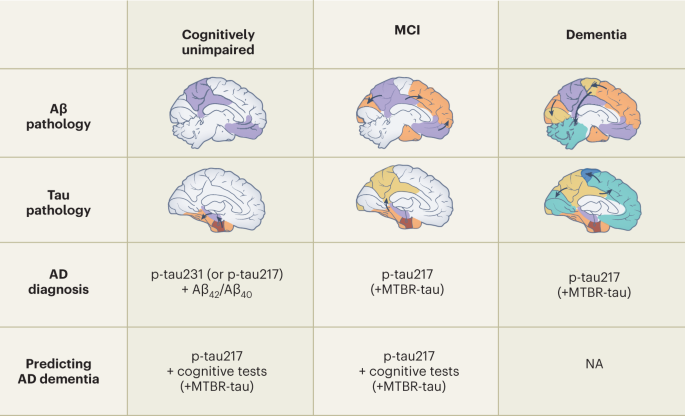
▷ Source: Nature Medicine